Helical hairpin structure of influenza hemagglutinin fusion peptide stabilized by charge-dipole interactions between the N-terminal amino group and the second helix
- PMID: 21319795
- PMCID: PMC3048900
- DOI: 10.1021/ja1099775
Helical hairpin structure of influenza hemagglutinin fusion peptide stabilized by charge-dipole interactions between the N-terminal amino group and the second helix
Abstract
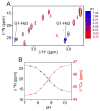
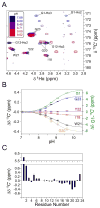
The fusion domain of the influenza coat protein hemagglutinin HA2, bound to dodecyl phosphocholine micelles, was recently shown to adopt a structure consisting of two antiparallel α-helices, packed in an exceptionally tight hairpin configuration. Four interhelical H(α) to C═O aliphatic H-bonds were identified as factors stabilizing this fold. Here, we report evidence for an additional stabilizing force: a strong charge-dipole interaction between the N-terminal Gly(1) amino group and the dipole moment of helix 2. pH titration of the amino-terminal (15)N resonance, using a methylene-TROSY-based 3D NMR experiment, and observation of Gly(1 13)C' show a strongly elevated pK = 8.8, considerably higher than expected for an N-terminal amino group in a lipophilic environment. Chemical shifts of three C-terminal carbonyl carbons of helix 2 titrate with the protonation state of Gly(1)-N, indicative of a close proximity between the N-terminal amino group and the axis of helix 2, providing an optimal charge-dipole stabilization of the antiparallel hairpin fold. pK values of the side-chain carboxylate groups of Glu(11) and Asp(19) are higher by about 1 and 0.5 unit, respectively, than commonly seen for solvent-exposed side chains in water-soluble proteins, indicative of dielectric constants of ε = ∼30 (Glu(11)) and ∼60 (Asp(19)), placing these groups in the headgroup region of the phospholipid micelle.
Figures

Similar articles
-
Closed and Semiclosed Interhelical Structures in Membrane vs Closed and Open Structures in Detergent for the Influenza Virus Hemagglutinin Fusion Peptide and Correlation of Hydrophobic Surface Area with Fusion Catalysis.J Am Chem Soc. 2015 Jun 24;137(24):7548-51. doi: 10.1021/jacs.5b04578. Epub 2015 Jun 10. J Am Chem Soc. 2015. PMID: 26039158 Free PMC article.
-
The complete influenza hemagglutinin fusion domain adopts a tight helical hairpin arrangement at the lipid:water interface.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11341-6. doi: 10.1073/pnas.1006142107. Epub 2010 Jun 2. Proc Natl Acad Sci U S A. 2010. PMID: 20534508 Free PMC article.
-
Structural characterizations of fusion peptide analogs of influenza virus hemagglutinin. Implication of the necessity of a helix-hinge-helix motif in fusion activity.J Biol Chem. 2002 Jun 21;277(25):22725-33. doi: 10.1074/jbc.M200089200. Epub 2002 Apr 5. J Biol Chem. 2002. PMID: 11937502
-
The impact of influenza hemagglutinin fusion peptide length and viral subtype on its structure and dynamics.Biopolymers. 2013 Mar;99(3):189-95. doi: 10.1002/bip.22102. Epub 2012 Sep 26. Biopolymers. 2013. PMID: 23015412 Free PMC article. Review.
-
Structural biology of the influenza virus fusion peptide.Acta Biochim Pol. 2014;61(3):421-6. Epub 2014 Sep 8. Acta Biochim Pol. 2014. PMID: 25195144 Review.
Cited by
-
Closed and Semiclosed Interhelical Structures in Membrane vs Closed and Open Structures in Detergent for the Influenza Virus Hemagglutinin Fusion Peptide and Correlation of Hydrophobic Surface Area with Fusion Catalysis.J Am Chem Soc. 2015 Jun 24;137(24):7548-51. doi: 10.1021/jacs.5b04578. Epub 2015 Jun 10. J Am Chem Soc. 2015. PMID: 26039158 Free PMC article.
-
The influenza hemagglutinin fusion domain is an amphipathic helical hairpin that functions by inducing membrane curvature.J Biol Chem. 2015 Jan 2;290(1):228-38. doi: 10.1074/jbc.M114.611657. Epub 2014 Nov 14. J Biol Chem. 2015. PMID: 25398882 Free PMC article.
-
Novel pH Selective, Highly Lytic Peptides Based on a Chimeric Influenza Hemagglutinin Peptide/Cell Penetrating Peptide Motif.Molecules. 2019 May 31;24(11):2079. doi: 10.3390/molecules24112079. Molecules. 2019. PMID: 31159194 Free PMC article.
-
Charged N-terminus of Influenza Fusion Peptide Facilitates Membrane Fusion.Int J Mol Sci. 2018 Feb 14;19(2):578. doi: 10.3390/ijms19020578. Int J Mol Sci. 2018. PMID: 29443945 Free PMC article.
-
Assembly of Influenza Hemagglutinin Fusion Peptides in a Phospholipid Bilayer by Coarse-grained Computer Simulations.Front Mol Biosci. 2015 Nov 18;2:66. doi: 10.3389/fmolb.2015.00066. eCollection 2015. Front Mol Biosci. 2015. PMID: 26636093 Free PMC article.
References
-
- Creighton TE. Proteins: structures and molecular properties. Macmillan; New York: 1993.
-
- Yohannan S, Faham S, Yang D, Grosfeld D, Chamberlain AK, Bowie JU. J Am Chem Soc. 2004;126:2284–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

