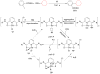
Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners
- PMID: 21319862
- PMCID: PMC3075866
- DOI: 10.1021/cr100327p
Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners
Figures
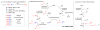












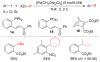

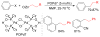







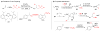
























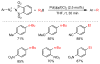








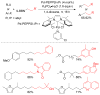







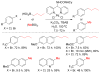
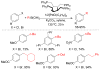

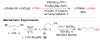


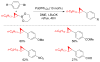





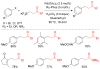


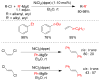
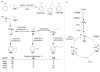




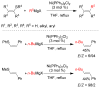


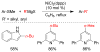


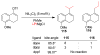








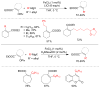


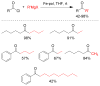



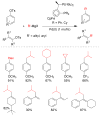
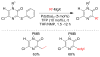


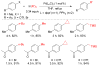
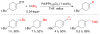



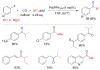








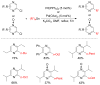














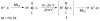
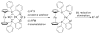


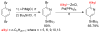











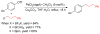



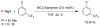





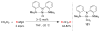
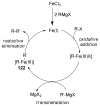



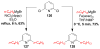



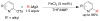
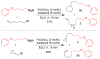

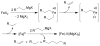











References
-
- de Meijere A, Diederich F, editors. In Metal-Catalyzed Cross-Coupling Reactions, Second Completely Revised and Enlarged Edition. Vol. 2. Wiley-VCH Verlag GmbH & Co; KGaA: 2004.
-
- de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions, Second, Completely Revised and Enlarged Edition. Vol. 1. Wiley-VC CH Verlag GmbH & Co; KGaA: 2004.
-
- Diederich F, Stang PJ, editors. Metal-catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinhein, Germany: 1998.
-
- Knochel P, Leuser H, Gong LZ, Perrone S, Kneisel FF. Chem Organozinc Compd. 2006:287.
-
- Knochel P, Leuser H, Gong L-Z, Perrone S, Kneisel FF. Polyfunctional zinc organometallics for organic synthesis. 2005;1
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

