Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients
- PMID: 21320152
- PMCID: PMC3141186
- DOI: 10.1111/j.1365-2125.2011.03940.x
Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients
Abstract
Aim: This study was designed to characterize the population pharmacokinetics of isoniazid in South African pulmonary tuberculosis patients.
Methods: Concentration-time measurements obtained from 235 patients receiving oral doses of isoniazid as part of routine tuberculosis chemotherapy in two clinical studies were pooled and subjected to nonlinear mixed-effects analysis.
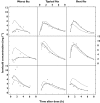
Results: A two-compartmental model, including first-order absorption and elimination with allometric scaling, was found to describe the observed dose-exposure relationship for oral isoniazid adequately. A mixture model was used to characterize dual rates of isoniazid elimination. Estimates of apparent clearance in slow and fast eliminators were 9.70 and 21.6 l h(-1) , respectively. The proportion of fast eliminators in the population was estimated to be 13.2%. Central volume of distribution was estimated to be 10% smaller in female patients and clearance was found to be 17% lower in patients with HIV. Variability in absorption rate (90%) was completely interoccasional in nature, whereas in relative bioavailability, interoccasional variability (8.4%) was lower than interindividual variability (26%). Oral doses, given once daily according to dosing policies at the time, were sufficient to reach therapeutic concentrations in the majority of the studied population, regardless of eliminator phenotype. Simulations suggested that current treatment guidelines (5 mg kg(-1) ) may be suboptimal in fast eliminators with low body weight.
Conclusions: A population pharmacokinetic model was developed to characterize the highly variable pharmacokinetics of isoniazid in a South African pulmonary tuberculosis patient population. Current treatment guidelines may lead to underexposure in rapid isoniazid eliminators.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures




References
-
- World Health Organization. Global Tuberculosis Control: WHO Report 2010. WHO/HTM/TB/2010.7. Geneva: World Health Organization; 2010.
-
- Wood R. The case for integrating tuberculosis and HIV treatment services in South Africa. J Infect Dis. 2007;196(Suppl 3):S497–499. - PubMed
-
- Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–49. - PubMed
-
- Donald PR, Sirgel FA, Botha FJ, Seifart HI, Parkin DP, Vandenplas ML, van de Wal BW, Maritz JS. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. - PubMed
-
- Donald PR, Sirgel FA, Venter A, Parkin DP, Seifart HI, van de Wal BW, Werely C, van Helden PD, Maritz JS. The influence of human N-acetyltransferase genotype on the early bactericidal activity of isoniazid. Clin Infect Dis. 2004;39:1425–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

