Effects of in vitro adult platelet transfusions on neonatal hemostasis
- PMID: 21320282
- PMCID: PMC3130591
- DOI: 10.1111/j.1538-7836.2011.04233.x
Effects of in vitro adult platelet transfusions on neonatal hemostasis
Abstract
Background: Thrombocytopenia is frequent among neonates, and 20-25% of affected infants are treated with platelet transfusions. These are frequently given for mild thrombocytopenia (platelets: 50-100 × 10(9) L(-1)), largely because of the known hyporeactivity of neonatal platelets. In tests of primary hemostasis, however, neonates have shorter bleeding and closure times (CTs) than adults. This has been attributed to their higher hematocrits, higher von Willebrand factor (VWF) concentrations, and predominance of longer VWF polymers.
Objective: To determine whether the 'transfusion' of adult (relatively hyperreactive) platelets into neonatal blood results in a hypercoagulable profile.
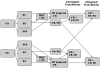
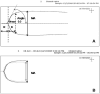
Methods: Cord blood (CB) and adult peripheral blood (PB) were separated (with a modified buffy coat method) to generate miniaturized platelet concentrates (PCs) and thrombocytopenic blood. PB-derived and CB-derived PCs (n = 7 per group) were then 'transfused'in vitro into thrombocytopenic CB and PB. The effects of autologous vs. allogeneic (developmentally mismatched) 'transfusions' were evaluated with whole blood aggregometry, a platelet function analyzer (PFA-100), and thromboelastography (TEG).
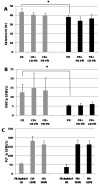
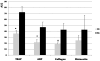
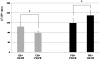
Results: Adult platelets aggregated significantly better than neonatal platelets in response to thrombin receptor-activating peptide, ADP, and collagen, regardless of the blood into which they were transfused. The 'transfusion' of adult platelets into thrombocytopenic CB resulted in shorter CTs-EPI (PFA-100) and higher clot strength and firmness (TEG) than 'transfusion' of neonatal autologous platelets.
Conclusions: In vitro'transfusion' of adult platelets into neonatal blood results in shorter CTs than 'transfusion' with neonatal platelets. Our findings should raise awareness of the differences between the neonatal and adult hemostatic system and the potential 'developmental mismatch' associated with platelet transfusions for neonatal hemostasis.
© 2011 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Figures

References
-
- Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749–55. - PubMed
-
- Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, Kiehn TI, Ainsworth S. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. 2006;26:348–53. - PubMed
-
- Del Vecchio A, Sola MC, Theriaque DW, Hutson AD, Kao KJ, Wright D, Garcia MG, Pollock BH, Christensen RD. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion. 2001;41:803–8. - PubMed
-
- Dohner ML, Wiedmeier SE, Stoddard RA, Null D, Jr, Lambert DK, Burnett J, Baer VL, Hunt JC, Henry E, Christensen RD. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion. 2009;49:869–72. - PubMed
-
- Josephson CD, Su LL, Christensen RD, Hillyer CD, Castillejo MI, Emory MR, Lin Y, Hume H, Easley K, Poterjoy B, Sola-Visner M. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics. 2009;123:278–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

