Efficacy and safety of a multifactor intervention to improve therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): protocol for the ICEPOC study
- PMID: 21320300
- PMCID: PMC3049740
- DOI: 10.1186/1745-6215-12-40
Efficacy and safety of a multifactor intervention to improve therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): protocol for the ICEPOC study
Abstract
Background: Low therapeutic adherence to medication is very common. Clinical effectiveness is related to dose rate and route of administration and so poor therapeutic adherence can reduce the clinical benefit of treatment. The therapeutic adherence of patients with chronic obstructive pulmonary disease (COPD) is extremely poor according to most studies. The research about COPD adherence has mainly focussed on quantifying its effect, and few studies have researched factors that affect non-adherence. Our study will evaluate the effectiveness of a multifactor intervention to improve the therapeutic adherence of COPD patients.
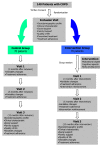
Methods/design: A randomized controlled clinical trial with 140 COPD diagnosed patients selected by a non-probabilistic method of sampling. Subjects will be randomly allocated into two groups, using the block randomization technique. Every patient in each group will be visited four times during the year of the study.
Intervention: Motivational aspects related to adherence (beliefs and behaviour): group and individual interviews; cognitive aspects: information about illness; skills: inhaled technique training. Reinforcement of the cognitive-emotional aspects and inhaled technique training will be carried out in all visits of the intervention group.
Discussion: Adherence to a prescribed treatment involves a behavioural change. Cognitive, emotional and motivational aspects influence this change and so we consider the best intervention procedure to improve adherence would be a cognitive and emotional strategy which could be applied in daily clinical practice. Our hypothesis is that the application of a multifactor intervention (COPD information, dose reminders and reinforcing audiovisual material, motivational aspects and inhalation technique training) to COPD patients taking inhaled treatment will give a 25% increase in the number of patients showing therapeutic adherence in this group compared to the control group.We will evaluate the effectiveness of this multifactor intervention on patient adherence to inhaled drugs considering that it will be right and feasible to the clinical practice context.
Trial registration: Current Controlled Trials ISRCTN18841601.
Figures
References
-
- World Health Organization. Adherence to long-term therapies: policy of action. Meeting report 4-5 June 2001. http://www.who.int/chronic-conditions/adherence/en/ (accessed may 2009)
-
- Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Respir Crit Care Med. 1995;152:580–8. - PubMed
-
- Kesten S, Flanders J, Serby C. Compliance with tiotropium, a once daily dry powder inhaled bronchodilator, in one year COPD trials. Chest. 2000;118:191S–2.
-
- van Grunsven PM, van Schayck CP, van Deuveren M, Herwaarden CL, Akkermans RP, van Weel C. Compliance during long term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. J Asthma. 2000;37:225–34. doi: 10.3109/02770900009055445. - DOI - PubMed