Mapping heat exchange in an allosteric protein
- PMID: 21320434
- PMCID: PMC3037712
- DOI: 10.1016/j.bpj.2010.12.3739
Mapping heat exchange in an allosteric protein
Abstract
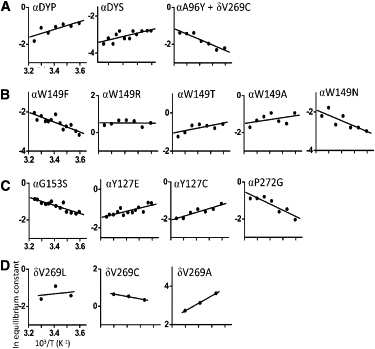
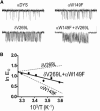
Nicotinic acetylcholine receptors (AChRs) are synaptic ion channels that spontaneously isomerize (i.e., gate) between resting and active conformations. We used single-molecule electrophysiology to measure the temperature dependencies of mouse neuromuscular AChR gating rate and equilibrium constants. From these we estimated free energy, enthalpy, and entropy changes caused by mutations of amino acids located between the transmitter binding sites and the middle of the membrane domain. The range of equilibrium enthalpy change (13.4 kcal/mol) was larger than for free energy change (5.5 kcal/mol at 25°C). For two residues, the slope of the rate-equilibrium free energy relationship (Φ) was approximately constant with temperature. Mutant cycle analysis showed that both free energies and enthalpies are additive for energetically independent mutations. We hypothesize that changes in energy associated with changes in structure mainly occur close to the site of the mutation, and, hence, that it is possible to make a residue-by-residue map of heat exchange in the AChR gating isomerization. The structural correlates of enthalpy changes are discussed for 12 different mutations in the protein.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. - PubMed
-
- Jackson M. Cambridge University Press; New York: 2006. Molecular and Cellular Biophysics.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

