Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications
- PMID: 21320489
- PMCID: PMC3110581
- DOI: 10.1016/j.expneurol.2011.02.004
Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications
Abstract
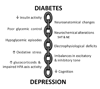
Diabetes mellitus is an endocrine disorder resulting from inadequate insulin release and/or reduced insulin sensitivity. The complications of diabetes are well characterized in peripheral tissues, but there is a growing appreciation that the complications of diabetes extend to the central nervous system (CNS). One of the potential neurological complications of diabetes is cognitive deficits. Interestingly, the structural, electrophysiological, neurochemical and anatomical underpinnings responsible for cognitive deficits in diabetes are strikingly similar to those observed in animals subjected to chronic stress, as well as in patients with stress-related psychiatric illnesses such as major depressive disorder. Since diabetes is a chronic metabolic stressor, this has led to the suggestion that common mechanistic mediators are responsible for neuroplasticity deficits in both diabetes and depression. Moreover, these common mechanistic mediators may be responsible for the increase in the risk of depressive illness in diabetes patients. In view of these observations, the aims of this review are (1) to describe the neuroplasticity deficits observed in diabetic rodents and patients; (2) to summarize the similarities in the clinical and preclinical studies of depression and diabetes; and (3) to highlight the diabetes-induced neuroplasticity deficits in those brain regions that have been implicated as important pathological centers in depressive illness, namely, the hippocampus, the amygdala and the prefrontal cortex.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Ajilore O, Haroon E, Kumaran S, Darwin C, Binesh N, Mintz J, Miller J, Thomas MA, Kumar A. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2007;32:1224–1231. - PubMed
-
- Akisaki T, Sakurai T, Takata T, Umegaki H, Araki A, Mizuno S, Tanaka S, Ohashi Y, Iguchi A, Yokono K, Ito H. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT) Diabetes Metab Res. Rev. 2006;22:376–384. - PubMed
-
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet. Med. 2006;23:1165–1173. - PubMed
-
- Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009;198:224–230. - PubMed
-
- Alzoubi KH, Aleisa AM, Alkadhi KA. Impairment of long-term potentiation in the CA1, but not dentate gyrus, of the hippocampus in Obese Zucker rats: role of calcineurin and phosphorylated CaMKII. J. Mol. Neurosci. 2005;27:337–346. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

