Gastrins, iron homeostasis and colorectal cancer
- PMID: 21320535
- PMCID: PMC3078979
- DOI: 10.1016/j.bbamcr.2011.02.007
Gastrins, iron homeostasis and colorectal cancer
Abstract
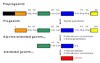
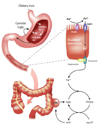
The peptide hormone gastrin has been identified as a major regulator of acid secretion and a potent mitogen for normal and malignant gastrointestinal cells. The importance of gastric acid in the absorption of dietary iron first became evident 50 years ago when iron deficiency anemia was recognized as a long-term consequence of partial gastrectomy. This review summarizes the connections between circulating gastrins, iron status and colorectal cancer. Gastrins bind two ferric ions with micromolar affinity and, in the case of non-amidated forms of the hormone, iron binding is essential for biological activity in vitro and in vivo. The demonstration of an interaction between gastrin and transferrin by biochemical techniques led to the proposal that gastrins catalyze the loading of transferrin with iron. Several lines of evidence, including the facts that the concentrations of circulating gastrins are increased in mice and humans with the iron overload disease hemochromatosis and that transferrin saturation positively correlates with circulating gastrin concentration, suggest the potential involvement of gastrins in iron homeostasis. Conversely, recognition that ferric ions play an unexpected role in the biological activity of gastrins may assist in the development of useful therapies for colorectal carcinoma and other disorders of mucosal proliferation in the gastrointestinal tract. This article is part of a Special Issue entitled: 11th European Symposium on Calcium.
2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Edkins JS. On the chemical mechanism of gastric secretion. Proc. Roy. Soc. B. 1905;76:376.
-
- Baird IM, Blackburn EK, Wilson GM. The pathogenesis of anaemia after partial gastrectomy. I. Development of anaemia in relation to time after operation, blood loss, and diet. Q J Med. 1959;28:21–34. - PubMed
-
- Baird IM, Wilson GM. The pathogenesis of anaemia after partial gastrectomy. II. Iron absorption after partial gastrectomy. Q J Med. 1959;28:35–41. - PubMed
-
- Baldwin GS, Curtain CC, Sawyer WH. Selective, high-affinity binding of ferric ions by glycine-extended gastrin(17) Biochemistry. 2001;40:10741–10746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

