Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303
- PMID: 21320619
- PMCID: PMC3150599
- DOI: 10.1016/j.bbmt.2011.02.002
Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303
Abstract
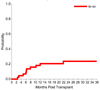
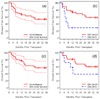
Graft-versus-host disease (GVHD) is most effectively prevented by ex vivo T cell depletion (TCD) of the allograft, but its role in the treatment of patients undergoing allogeneic hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in complete remission (CR) remains unclear. We performed a phase 2 single-arm multicenter study to evaluate the role of TCD in AML patients in CR1 or CR2 up to age 65 years. The primary objective was to achieve a disease-free survival (DFS) rate of >75% at 6 months posttransplantation. A total of 44 patients with AML in CR1 (n = 37) or CR2 (n = 7) with a median age of 48.5 years (range, 21-59 years) received myeloablative chemotherapy and fractionated total body irradiation (1375 cGy) followed by immunomagnetically selected CD34-enriched, T cell‒depleted allografts from HLA-identical siblings. No pharmacologic GVHD prophylaxis was given. All patients engrafted. The incidence of acute GVHD grade II-IV was 22.7%, and the incidence of extensive chronic GVHD was 6.8% at 24 months. The relapse rate for patients in CR1 was 17.4% at 36 months. With a median follow-up of 34 months, DFS for all patients was 82% at 6 months, and DFS for patients in CR1 was 72.8% at 12 months and 58% at 36 months. HCT after myeloablative chemoradiotherapy can be performed in a multicenter setting using a uniform method of TCD, resulting in a low risk of extensive chronic GVHD and relapse for patients with AML in CR1.
Copyright © 2011 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
S.M.D., S.C., R.J.S., and R.J.O. have served as advisors to Miltenyi Biotec Inc
Figures


Comment in
-
Ex vivo T cell depletion of allogeneic PBSC as acute and chronic GVHD prophylaxis after myeloablative HCT: time to reconsider?Biol Blood Marrow Transplant. 2011 Aug;17(8):1112-3. doi: 10.1016/j.bbmt.2011.02.005. Epub 2011 Mar 5. Biol Blood Marrow Transplant. 2011. PMID: 21382501 No abstract available.
References
-
- Copelan EA. Hematopoietic Stem-Cell Transplantation. N Engl J Med. 2006;354:1813–1826. - PubMed
-
- Cornelissen JJ, van Putten WLJ, Verdonck LF, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–3666. - PubMed
-
- Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. - PubMed
-
- Suciu S, Mandelli F, de Witte T, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003;102:1232–1240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

