Redox homeostasis, oxidative stress and disuse muscle atrophy
- PMID: 21320887
- PMCID: PMC3098694
- DOI: 10.1113/jphysiol.2010.203232
Redox homeostasis, oxidative stress and disuse muscle atrophy
Abstract
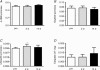
A pivotal role has been ascribed to oxidative stress in determining the imbalance between protein synthesis and degradation leading to muscle atrophy in many pathological conditions and in disuse. However, a large variability in disuse-induced alteration of redox homeostasis through muscles, models and species emerges from the literature. Whereas the causal role of oxidative stress appears well established in the mechanical ventilation model, findings are less compelling in the hindlimb unloaded mice and very limited in humans. The mere coexistence of muscle atrophy, indirect indexes of increased reactive oxygen species (ROS) production and impairment of antioxidant defence systems, in fact, does not unequivocally support a causal role of oxidative stress in the phenomenon. We hypothesise that in some muscles, models and species only, due to a large redox imbalance, the leading phenomena are activation of proteolysis and massive oxidation of proteins, which would become more susceptible to degradation. In other conditions, due to a lower extent and variable time course of ROS production, different ROS-dependent, but also -independent intracellular pathways might dominate determining the variable extent of atrophy and even dispensable protein oxidation. The ROS production and removal are complex and finely tuned phenomena. They are indeed important intracellular signals and redox balance maintains normal muscle homeostasis and can underlie either positive or negative adaptations to exercise. A precise approach to determine the levels of ROS in living cells in various conditions appears to be of paramount importance to define and support such hypotheses.
Figures
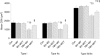

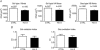


References
-
- Aguilo A, Tauler P, Fuentespina E, Tur JA, Cordova A, Pons A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav. 2005;84:1–7. - PubMed
-
- Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp Neurol. 1987;96:635–649. - PubMed
-
- Appell HJ, Duarte JA, Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–160. - PubMed
-
- Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102:956–964. - PubMed
-
- Baldwin KM. Effect of spaceflight on the functional, biochemical, and metabolic properties of skeletal muscle. Med Sci Sports Exerc. 1996;28:983–987. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

