Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: underlying mechanisms
- PMID: 21320889
- PMCID: PMC3090600
- DOI: 10.1113/jphysiol.2010.202044
Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: underlying mechanisms
Abstract
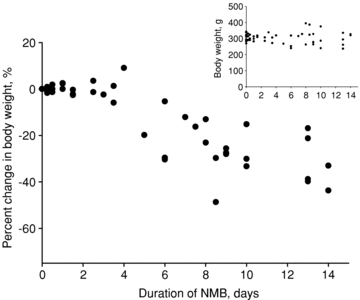
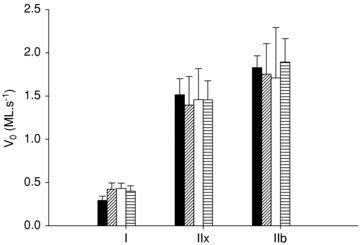
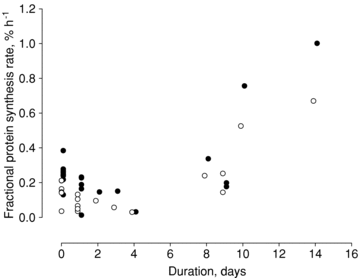
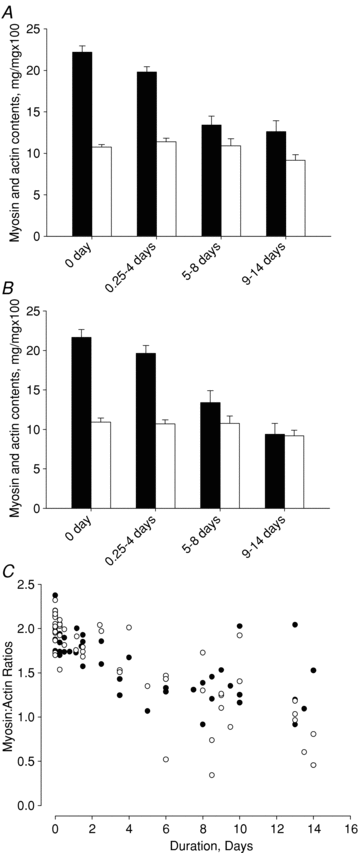
The muscle wasting and impaired muscle function in critically ill intensive care unit (ICU) patients delay recovery from the primary disease, and have debilitating consequences that can persist for years after hospital discharge. It is likely that, in addition to pernicious effects of the primary disease, the basic life support procedures of long-term ICU treatment contribute directly to the progressive impairment of muscle function. This study aims at improving our understanding of the mechanisms underlying muscle wasting in ICU patients by using a unique experimental rat ICU model where animals are mechanically ventilated, sedated and pharmacologically paralysed for duration varying between 6 h and 14 days. Results show that the ICU intervention induces a phenotype resembling the severe muscle wasting and paralysis associated with the acute quadriplegic myopathy (AQM) observed in ICU patients, i.e. a preferential loss of myosin, transcriptional down-regulation of myosin synthesis, muscle atrophy and a dramatic decrease in muscle fibre force generation capacity. Detailed analyses of protein degradation pathways show that the ubiquitin proteasome pathway is highly involved in this process. A sequential change in localisation of muscle-specific RING finger proteins 1/2 (MuRF1/2) observed during the experimental period is suggested to play an instrumental role in both transcriptional regulation and protein degradation. We propose that, for those critically ill patients who develop AQM, complete mechanical silencing, due to pharmacological paralysis or sedation, is a critical factor underlying the preferential loss of the molecular motor protein myosin that leads to impaired muscle function or persisting paralysis.
Figures




References
-
- Ahlbeck K, Fredriksson K, Rooyackers O, Maback G, Remahl S, Ansved T, et al. Signs of critical illness polyneuropathy and myopathy can be seen early in the ICU course. Acta Anaesthesiol Scand. 2009;53:717–723. - PubMed
-
- Balagopal P, Ford GC, Ebenstein DB, Nadeau DA, Nair KS. Mass spectrometric methods for determination of [13C]leucine enrichment in human muscle protein. Anal Biochem. 1996;239:77–85. - PubMed
-
- Banduseela VC, Ochala J, Chen YW, Goransson H, Norman H, Radell P, et al. Gene expression and muscle fiber function in a porcine ICU model. Physiol Genomics. 2009;39:141–159. - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. - PubMed
-
- Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, et al. Two year outcomes, health care use and costs in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

