Quantitative analysis reveals multiple mechanisms of allosteric modulation of the mGlu5 receptor in rat astroglia
- PMID: 21321061
- PMCID: PMC3082933
- DOI: 10.1124/mol.110.068882
Quantitative analysis reveals multiple mechanisms of allosteric modulation of the mGlu5 receptor in rat astroglia
Abstract
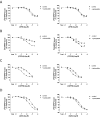
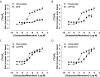
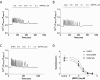
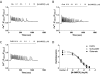
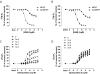
Positive and negative allosteric modulators (PAMs and NAMs, respectively) of the type 5 metabotropic glutamate (mGlu5) receptor have demonstrable therapeutic potential in an array of neurological and psychiatric disorders. Here, we have used rat cortical astrocytes to investigate how PAMs and NAMs mediate their activity and reveal marked differences between PAMs with respect to their modulation of orthosteric agonist affinity and efficacy. Affinity cooperativity factors (α) were assessed using [(3)H]2-methyl-6-(phenylethynyl)-pyridine (MPEP)-PAM competition binding in the absence and presence of orthosteric agonist, whereas efficacy cooperativity factors (β) were calculated from net affinity/efficacy cooperativity parameters (αβ) obtained from analyses of the abilities of PAMs to potentiate [(3)H]inositol phosphate accumulation in astrocytes stimulated with a submaximal (EC(20)) concentration of orthosteric agonist. We report that whereas 3,3'-difluorobenzaldazine (DFB) and 3-cyano-N-(1,3-diphenyl-1H-prazol-5-yl)benzamide (CDPPB) primarily exert their allosteric modulatory effects through modifying the apparent orthosteric agonist affinity at the astrocyte mGlu5 receptor, the effects of S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidinl-1-yl}-methanone (ADX47273) are mediated primarily via efficacy-driven modulation. In [(3)H]MPEP-NAM competition binding assays, both MPEP and 2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine (M-5MPEP) defined similar specific binding components, with affinities that were unaltered in the presence of orthosteric agonist, indicating wholly negative efficacy-driven modulations. It is noteworthy that whereas M-5MPEP only partially inhibited orthosteric agonist-stimulated [(3)H]inositol phosphate accumulation in astrocytes, it could completely suppress Ca(2+) oscillations stimulated by quisqualate or (S)-3,5-dihydroxyphenylglycine. In contrast, MPEP was fully inhibitory with respect to both functional responses. The finding that M-5MPEP has different functional effects depending on the endpoint measured is discussed as a possible example of permissive allosteric antagonism.
Figures
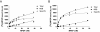

References
-
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford ND, Varney MA. (2002) [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther 303:1044–1051 - PubMed
-
- Biber K, Laurie DJ, Berthele A, Sommer B, Tölle TR, Gebicke-Härter PJ, van Calker D, Boddeke HW. (1999) Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem 72:1671–1680 - PubMed
-
- Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. (2008) Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology 55:392–395 - PubMed
-
- Chen Y, Goudet C, Pin JP, Conn PJ. (2008) N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73:909–918 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

