Role for miR-204 in human pulmonary arterial hypertension
- PMID: 21321078
- PMCID: PMC3058572
- DOI: 10.1084/jem.20101812
Role for miR-204 in human pulmonary arterial hypertension
Abstract
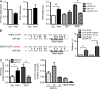
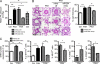
Pulmonary arterial hypertension (PAH) is characterized by enhanced proliferation and reduced apoptosis of pulmonary artery smooth muscle cells (PASMCs). Because microRNAs have been recently implicated in the regulation of cell proliferation and apoptosis, we hypothesized that these regulatory molecules might be implicated in the etiology of PAH. In this study, we show that miR-204 expression in PASMCs is down-regulated in both human and rodent PAH. miR-204 down-regulation correlates with PAH severity and accounts for the proliferative and antiapoptotic phenotypes of PAH-PASMCs. STAT3 activation suppresses miR-204 expression, and miR-204 directly targets SHP2 expression, thereby SHP2 up-regulation, by miR-204 down-regulation, activates the Src kinase and nuclear factor of activated T cells (NFAT). STAT3 also directly induces NFATc2 expression. NFAT and SHP2 were needed to sustain PAH-PASMC proliferation and resistance to apoptosis. Finally, delivery of synthetic miR-204 to the lungs of animals with PAH significantly reduced disease severity. This study uncovers a new regulatory pathway involving miR-204 that is critical to the etiology of PAH and indicates that reestablishing miR-204 expression should be explored as a potential new therapy for this disease.
Figures





References
-
- Archer S., Rich S. 2000. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation. 102:2781–2791 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

