Sagopilone (ZK-EPO, ZK 219477) for recurrent glioblastoma. A phase II multicenter trial by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group
- PMID: 21321091
- PMCID: PMC3164435
- DOI: 10.1093/annonc/mdq729
Sagopilone (ZK-EPO, ZK 219477) for recurrent glioblastoma. A phase II multicenter trial by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group
Abstract
Background: Sagopilone (ZK 219477), a lipophylic and synthetic analog of epothilone B, that crosses the blood-brain barrier has demonstrated preclinical activity in glioma models.
Patients and methods: Patients with first recurrence/progression of glioblastoma were eligible for this early phase II and pharmacokinetic study exploring single-agent sagopilone (16 mg/m(2) over 3 h every 21 days). Primary end point was a composite of either tumor response or being alive and progression free at 6 months. Overall survival, toxicity and safety and pharmacokinetics were secondary end points.
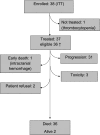
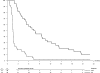
Results: Thirty-eight (evaluable 37) patients were included. Treatment was well tolerated, and neuropathy occurred in 46% patients [mild (grade 1) : 32%]. No objective responses were seen. The progression-free survival (PFS) rate at 6 months was 6.7% [95% confidence interval (CI) 1.3-18.7], the median PFS was just over 6 weeks, and the median overall survival was 7.6 months (95% CI 5.3-12.3), with a 1-year survival rate of 31.6% (95% CI 17.7-46.4). Maximum plasma concentrations were reached at the end of the 3-h infusion, with rapid declines within 30 min after termination.
Conclusions: No evidence of relevant clinical antitumor activity against recurrent glioblastoma could be detected. Sagopilone was well tolerated, and moderate-to-severe peripheral neuropathy was observed in despite prolonged administration.
Figures
References
-
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- BarkerII FG, II, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709–720. discussion 720–723. - PubMed
-
- Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE Study. J Clin Oncol. 2010;28:2051–2057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials