A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves' ophthalmopathy
- PMID: 21321093
- PMCID: PMC3074639
- DOI: 10.1530/JME-11-0006
A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves' ophthalmopathy
Abstract
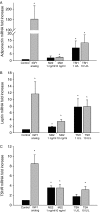
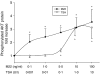
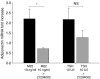
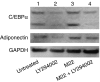
Graves' ophthalmopathy (GO) is characterized by expanded volume of the orbital tissues associated with elevated serum levels of TSH receptor (TSHR) autoantibodies. Because previous studies have demonstrated evidence of adipogenesis within the GO orbit, we sought to determine whether M22, a human monoclonal antibody directed against TSHR, enhances adipogenesis in orbital fibroblasts from patients with GO and, if so, to identify signaling mechanisms involved. GO orbital fibroblast cultures (n=10) were treated for 10 days with bovine TSH (1 or 10.0 U/l) or M22 (1 or 10 ng/ml) in serum-free adipocyte differentiation medium. Some cultures also received a phosphoinositide 3-kinase (PI3K) inhibitor or an inhibitor of cAMP production. In other experiments, confluent cultures (n=8) were treated for between 1 and 30 min with TSH (0.1-10.0 U/l) or M22 (0.1-100 ng/ml) with measurement of cAMP production or levels of phosphorylated AKT (pAKT). We found levels of adiponectin, leptin, and TSHR mRNA to be increased in GO cultures treated for 10 days with either M22 (2.6 mean fold ± 0.7; P=0.03) or TSH (13.2 ± 5.8-fold, P=0.048). In other studies, M22 and TSH stimulated cAMP production and pAKT levels in GO cells. Inhibition of PI3K activity during 10 days in culture decreased the levels of M22-stimulated mRNA encoding adiponectin (67 ± 12%; P=0.021), as well as adiponectin and CCAAT/enhancer-binding protein α protein levels. In conclusion, M22 is a pro-adipogenic factor in GO orbital preadipocytes. This antibody appears to act via the PI3K signaling cascade, suggesting that inhibition of PI3K signaling may represent a potential novel therapeutic approach in GO.
Figures



References
-
- Crisp M, Starkey K, Lane C, Ham J, Ludgate M. Adipogenesis in thyroid eye disease. Investigative Ophthalmology and Visual Science. 2000;41:3249–3255. - PubMed
-
- Eckstein AK, Plicht M, Lax H, Neuhauser M, Mann K, Lederbogen S, Heckmann C, Esser J, Morgenthaler NG. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. Journal of Clinical Endocrinology and Metabolism. 2006;91:3464–3470. doi: 10.1210/jc.2005-2813. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

