Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response
- PMID: 21321124
- PMCID: PMC3064169
- DOI: 10.1074/jbc.M110.217349
Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response
Abstract
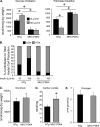
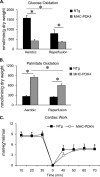
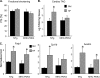
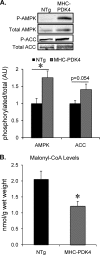
Diabetic cardiac dysfunction is associated with decreased rates of myocardial glucose oxidation (GO) and increased fatty acid oxidation (FAO), a fuel shift that has been shown to sensitize the heart to ischemic insult and ventricular dysfunction. We sought to evaluate the metabolic and functional consequences of chronic suppression of GO in heart as modeled by transgenic mice with cardiac-specific overexpression of pyruvate dehydrogenase kinase 4 (myosin heavy chain (MHC)-PDK4 mice), an inhibitor of pyruvate dehydrogenase. Hearts of MHC-PDK4 mice were shown to exhibit an insulin-resistant substrate utilization profile, characterized by low GO rates and high FAO flux. Surprisingly, MHC-PDK4 mice were not sensitized to cardiac ischemia-reperfusion injury despite a fuel utilization pattern that phenocopied the diabetic heart. In addition, MHC-PDK4 mice were protected against high fat diet-induced myocyte lipid accumulation, likely related to increased capacity for FAO. The high rates of mitochondrial FAO in the MHC-PDK4 heart were related to heightened activity of the AMP-activated protein kinase, reduced levels of malonyl-CoA, and increased capacity for mitochondrial uncoupled respiration. The expression of the known AMP-activated protein kinase target, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a master regulator of mitochondrial function and biogenesis, was also activated in the MHC-PDK4 heart. These results demonstrate that chronic activation of PDK4 triggers transcriptional and post-transcriptional mechanisms that re-program the heart for chronic high rates of FAO without the expected deleterious functional or metabolic consequences.
Figures

References
-
- Engelgau M. M., Geiss L. S., Saaddine J. B., Boyle J. P., Benjamin S. M., Gregg E. W., Tierney E. F., Rios-Burrows N., Mokdad A. H., Ford E. S., Imperatore G., Narayan K. M. (2004) Ann. Intern. Med. 140, 945–950 - PubMed
-
- Zimmet P., Alberti K. G., Shaw J. (2001) Nature 414, 782–787 - PubMed
-
- Fein F. S., Sonnenblick E. H. (1985) Prog. Cardiovasc. Dis. 27, 255–270 - PubMed
-
- Rubler S., Dlugash J., Yuceoglu Y. Z., Kumral T., Branwood A. W., Grishman A. (1972) Am. J. Cardiol. 30, 595–602 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

