Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients
- PMID: 21321214
- PMCID: PMC3048107
- DOI: 10.1073/pnas.1014835108
Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients
Abstract
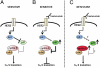
Clinical benefits from trastuzumab and other anti-HER2 therapies in patients with HER2 amplified breast cancer remain limited by primary or acquired resistance. To identify potential mechanisms of resistance, we established trastuzumab-resistant HER2 amplified breast cancer cells by chronic exposure to trastuzumab treatment. Genomewide copy-number variation analyses of the resistant cells compared with parental cells revealed a focal amplification of genomic DNA containing the cyclin E gene. In a cohort of 34 HER2(+) patients treated with trastuzumab-based therapy, we found that cyclin E amplification/overexpression was associated with a worse clinical benefit (33.3% compared with 87.5%, P < 0.02) and a lower progression-free survival (6 mo vs. 14 mo, P < 0.002) compared with nonoverexpressing cyclin E tumors. To dissect the potential role of cyclin E in trastuzumab resistance, we studied the effects of cyclin E overexpression and cyclin E suppression. Cyclin E overexpression resulted in resistance to trastuzumab both in vitro and in vivo. Inhibition of cyclin E activity in cyclin E-amplified trastuzumab resistant clones, either by knockdown of cyclin E expression or treatment with cyclin-dependent kinase 2 (CDK2) inhibitors, led to a dramatic decrease in proliferation and enhanced apoptosis. In vivo, CDK2 inhibition significantly reduced tumor growth of trastuzumab-resistant xenografts. Our findings point to a causative role for cyclin E overexpression and the consequent increase in CDK2 activity in trastuzumab resistance and suggest that treatment with CDK2 inhibitors may be a valid strategy in patients with breast tumors with HER2 and cyclin E coamplification/overexpression.
Conflict of interest statement
Conflict of interest statement: S.R.G. is an employee of Cyclacel, Ltd.
Figures


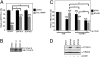

References
-
- Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. - PubMed
-
- Slamon DJ, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

