Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study
- PMID: 21321298
- PMCID: PMC3083867
- DOI: 10.1200/JCO.2010.31.6455
Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study
Abstract
Purpose: Among postmenopausal women with endocrine-responsive breast cancer, the aromatase inhibitor letrozole, when compared with tamoxifen, has been shown to significantly improve disease-free survival (DFS) and time to distant recurrence (TDR). We investigated whether letrozole monotherapy prolonged overall survival (OS) compared with tamoxifen monotherapy.
Patients and methods: Of 8,010 postmenopausal women with hormone receptor-positive, early breast cancer enrolled on the Breast International Group (BIG) 1-98 study, 4,922 were randomly assigned to 5 years of continuous adjuvant therapy with either letrozole or tamoxifen. Of 2,459 patients enrolled in the tamoxifen treatment arm, 619 (25.2%) selectively crossed over to either adjuvant or extended letrozole after initial trial results were presented in January 2005. To gain better estimates of relative treatment effects in the presence of selective crossover, we used inverse probability of censoring weighted (IPCW) modeling.
Results: Weighted Cox models, by using IPCW, estimated a statistically significant, 18% reduction in the hazard of an OS event with letrozole treatment (hazard ratio [HR], 0.82; 95% CI, 0.70 to 0.95). Estimates of 5-year OS on the basis of IPCW were 91.8% and 90.4% for letrozole and tamoxifen, respectively. The HRs of DFS and TDR events by using IPCW modeling were 0.83 (95% CI, 0.74 to 0.94) and 0.80 (95% CI, 0.67 to 0.94), respectively (P < .05 for DFS, OS, and TDR). Median follow-up was 74 months.
Conclusion: Adjuvant treatment with letrozole, compared with tamoxifen, significantly reduces the risk of death, the risk of recurrent disease, and the risk of recurrence at distant sites in postmenopausal women with hormone receptor-positive breast cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


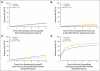
Comment in
-
Correcting for discretionary treatment crossover in an analysis of survival in the Breast International Group BIG 1-98 trial by using the inverse probability of censoring weighted method.J Clin Oncol. 2011 Mar 20;29(9):1093-5. doi: 10.1200/JCO.2010.33.9374. Epub 2011 Feb 14. J Clin Oncol. 2011. PMID: 21321297 No abstract available.
-
Medical oncology: Letrozole efficacy confirmed.Nat Rev Clin Oncol. 2011 May;8(5):253. doi: 10.1038/nrclinonc.2011.39. Nat Rev Clin Oncol. 2011. PMID: 21451465 No abstract available.
References
-
- Breast International Group 1-98 Collaborative Group. Thürlimann B, Keshaviah A, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. - PubMed
-
- Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. - PubMed
-
- Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. - PubMed
-
- Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 study. J Clin Oncol. 2007;25:2664–2670. - PubMed
-
- Boccardo F. Switching to anastrozole after tamoxifen improves survival in postmenopausal women with breast cancer. Nat Clin Pract Oncol. 2008;5:76–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

