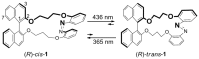
Synthesis and conformation of substituted chiral binaphthyl-azobenzene cyclic dyads with chiroptical switching capabilities
- PMID: 21321531
- PMCID: PMC6259615
- DOI: 10.3390/molecules16021603
Synthesis and conformation of substituted chiral binaphthyl-azobenzene cyclic dyads with chiroptical switching capabilities
Abstract
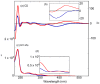
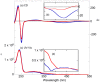
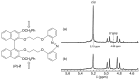
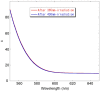
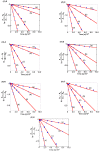
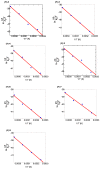
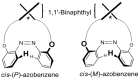
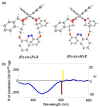
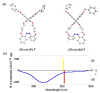
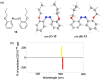
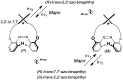
Optically active binaphthyl-azobenezene cyclic dyads were synthesized to develop a photochromic switching molecule. Azobenezene moieties were cis-trans isomerized by photoirradiation. As a reflection of the structural change, the specific optical rotation and circular dichroism underwent significant shifts. Under certain conditions, the positive-negative and zero-positive (or zero-negative) signals were reversed. Optical rotation may potentially be applied in noise-cancelling nondestructive photoswiches. The conformations were studied by experimental and theoretical methods. The results revealed that the helical chirality, (P) or (M), of the cis-azobenzene moiety was induced by intramolecular axial chirality. The twist direction depended on the axial chirality as well as the azobenzene linkage position to the binaphthyls, but was independent of the identity of substituted groups. 2,2'-Linked-(R)-binaphthyl was found to induce cis-(P)-azobenzene, whereas symmetrically 7,7'-linked-(R)-binaphthyl was found to induce cis-(M)-azobenzene.
Figures



Similar articles
-
Photoinversion of cisoid/transoid binaphthyls.Org Lett. 2012 Jan 6;14(1):276-9. doi: 10.1021/ol203053q. Epub 2011 Dec 13. Org Lett. 2012. PMID: 22148882
-
A versatile cyclic 2,2'-azobenzenophane with a functional handle and its polymers: efficient synthesis and effect of topological structure on chiroptical properties.Chemistry. 2015 Feb 2;21(6):2324-9. doi: 10.1002/chem.201405940. Epub 2014 Dec 15. Chemistry. 2015. PMID: 25510346
-
Fusion of photochromic reaction and synthetic reaction: photoassisted cyclization to highly strained chiral azobenzenophanes.Org Lett. 2012 Jul 6;14(13):3252-5. doi: 10.1021/ol300992d. Epub 2012 Jun 13. Org Lett. 2012. PMID: 22694191
-
Computing chiroptical properties with first-principles theoretical methods: background and illustrative examples.Chirality. 2009;21 Suppl 1:E116-52. doi: 10.1002/chir.20789. Chirality. 2009. PMID: 20014411 Review.
-
Azobenzene as photoresponsive conformational switch in cyclic peptides.J Pept Res. 2005 Jan;65(1):4-14. doi: 10.1111/j.1399-3011.2004.00203.x. J Pept Res. 2005. PMID: 15686529 Review.
Cited by
-
Directing Coupled Motion with Light: A Key Step Toward Machine-Like Function.Chem Rev. 2021 Nov 10;121(21):13213-13237. doi: 10.1021/acs.chemrev.1c00340. Epub 2021 Sep 17. Chem Rev. 2021. PMID: 34533944 Free PMC article.
-
Covalent functionalization of graphene by azobenzene with molecular hydrogen bonds for long-term solar thermal storage.Sci Rep. 2013 Nov 19;3:3260. doi: 10.1038/srep03260. Sci Rep. 2013. PMID: 24247355 Free PMC article.
References
-
- Zheng Y., Cui J., Zheng J., Wan X. Near-infrared electrochromic and chiroptical switching polymers: Synthesis and characterization of helical poly(N-propargylamides) carrying anthraquinone imide moieties in side chains. J. Mater. Chem. 2010;20:5915–5922. doi: 10.1039/b927360c. - DOI
-
- Li D., Wang Z.Y., Ma D. Electrically-controlled near-infrared chiroptical switching of enantiomeric dinuclear ruthenium complexes. Chem. Commun. 2009:1529–1531. - PubMed
-
- Deng J., Zhou C., Chen C., Song N., Su Z. Synthesis and redox-driven chiroptically switching properties of viologen-containing optically active polymer with main-chain axial chirality. Macromolecules. 2008;41:7805–7811. doi: 10.1021/ma8014646. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

