Regulation and function of DNA methylation in plants and animals
- PMID: 21321601
- PMCID: PMC3152208
- DOI: 10.1038/cr.2011.23
Regulation and function of DNA methylation in plants and animals
Abstract
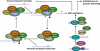
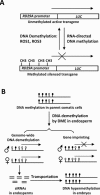
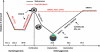
DNA methylation is an important epigenetic mark involved in diverse biological processes. In plants, DNA methylation can be established through the RNA-directed DNA methylation pathway, an RNA interference pathway for transcriptional gene silencing (TGS), which requires 24-nt small interfering RNAs. In mammals, de novo DNA methylation occurs primarily at two developmental stages: during early embryogenesis and during gametogenesis. While it is not clear whether establishment of DNA methylation patterns in mammals involves RNA interference in general, de novo DNA methylation and suppression of transposons in germ cells require 24-32-nt piwi-interacting small RNAs. DNA methylation status is dynamically regulated by DNA methylation and demethylation reactions. In plants, active DNA demethylation relies on the repressor of silencing 1 family of bifunctional DNA glycosylases, which remove the 5-methylcytosine base and then cleave the DNA backbone at the abasic site, initiating a base excision repair (BER) pathway. In animals, multiple mechanisms of active DNA demethylation have been proposed, including a deaminase- and DNA glycosylase-initiated BER pathway. New information concerning the effects of various histone modifications on the establishment and maintenance of DNA methylation has broadened our understanding of the regulation of DNA methylation. The function of DNA methylation in plants and animals is also discussed in this review.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

