Prostate tumor cells with cancer progenitor properties have high telomerase activity and are rapidly killed by telomerase interference
- PMID: 21321978
- PMCID: PMC3123672
- DOI: 10.1002/pros.21355
Prostate tumor cells with cancer progenitor properties have high telomerase activity and are rapidly killed by telomerase interference
Abstract
Background: Cancer progenitor cells (CPCs) have been postulated to promote treatment resistance and disease progression in prostate and other malignancies. We investigated whether the enzyme telomerase, which is active in cancer cells and in normal stem cells, plays an important role in CPC which can be exploited to neutralize these cells.
Methods: We used flow cytometry and assays of gene expression, clonogenicity, and invasiveness to isolate and characterize a putative CPC subpopulation from freshly resected human prostatectomy specimens. Telomerase activity was measured by qPCR-based Telomeric Repeat Amplification Protocol (TRAP). Telomerase interference was achieved by ectopic expression of a mutated telomerase RNA construct which reprograms telomerase to generate "toxic" uncapped telomeres. Treated cells were assayed for apoptosis, proliferation in culture, and xenograft tumor formation.
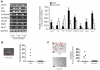
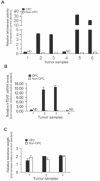
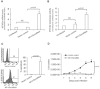
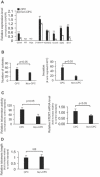
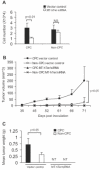
Results: CPC in prostate tumors expressed elevated levels of genes associated with a progenitor phenotype and were highly clonogenic and invasive. Significantly, CPC telomerase activity was 20- to 200-fold higher than in non-CPC from the same tumors, and CPC were exquisitely sensitive to telomerase interference which induced rapid apoptosis and growth inhibition. Similarly, induction of telomerase interference in highly tumorigenic CPC isolated from a prostate cancer cell line abrogated their ability to form tumor xenografts.
Conclusions: Human prostate tumors contain a CPC subpopulation with markedly elevated telomerase activity which renders them acutely susceptible to telomerase interference. These findings offer the first tumor-derived and in vivo evidence that telomerase may constitute a CPC "Achilles heel" which may ultimately form the basis for more effective new CPC-targeting therapies.
Copyright © 2011 Wiley-Liss, Inc.
Figures
References
-
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15(9):1010–1012. - PubMed
-
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. - PubMed
-
- Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114(Pt 21):3865–3872. - PubMed
-
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

