Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity
- PMID: 21323540
- PMCID: PMC3119530
- DOI: 10.1056/NEJMoa1007374
Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity
Abstract
Background: Retinopathy of prematurity is a leading cause of childhood blindness worldwide. Peripheral retinal ablation with conventional (confluent) laser therapy is destructive, causes complications, and does not prevent all vision loss, especially in cases of retinopathy of prematurity affecting zone I of the eye. Case series in which patients were treated with vascular endothelial growth factor inhibitors suggest that these agents may be useful in treating retinopathy of prematurity.
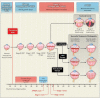
Methods: We conducted a prospective, controlled, randomized, stratified, multicenter trial to assess intravitreal bevacizumab monotherapy for zone I or zone II posterior stage 3+ (i.e., stage 3 with plus disease) retinopathy of prematurity. Infants were randomly assigned to receive intravitreal bevacizumab (0.625 mg in 0.025 ml of solution) or conventional laser therapy, bilaterally. The primary ocular outcome was recurrence of retinopathy of prematurity in one or both eyes requiring retreatment before 54 weeks' postmenstrual age.
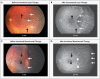
Results: We enrolled 150 infants (total sample of 300 eyes); 143 infants survived to 54 weeks' postmenstrual age, and the 7 infants who died were not included in the primary-outcome analyses. Retinopathy of prematurity recurred in 4 infants in the bevacizumab group (6 of 140 eyes [4%]) and 19 infants in the laser-therapy group (32 of 146 eyes [22%], P=0.002). A significant treatment effect was found for zone I retinopathy of prematurity (P=0.003) but not for zone II disease (P=0.27).
Conclusions: Intravitreal bevacizumab monotherapy, as compared with conventional laser therapy, in infants with stage 3+ retinopathy of prematurity showed a significant benefit for zone I but not zone II disease. Development of peripheral retinal vessels continued after treatment with intravitreal bevacizumab, but conventional laser therapy led to permanent destruction of the peripheral retina. This trial was too small to assess safety. (Funded by Research to Prevent Blindness and others; ClinicalTrials.gov number, NCT00622726.).
Figures

Comment in
-
Bevacizumab for retinopathy of prematurity.N Engl J Med. 2011 Feb 17;364(7):677-8. doi: 10.1056/NEJMe1100248. N Engl J Med. 2011. PMID: 21323546 No abstract available.
-
Bevacizumab for retinopathy of prematurity.N Engl J Med. 2011 Jun 16;364(24):2360; author reply 2361-2. doi: 10.1056/NEJMc1103460. N Engl J Med. 2011. PMID: 21675897 No abstract available.
-
Bevacizumab for retinopathy of prematurity.N Engl J Med. 2011 Jun 16;364(24):2359-60; author reply 2361-2. doi: 10.1056/NEJMc1103460. N Engl J Med. 2011. PMID: 21675898 No abstract available.
-
Bevacizumab for retinopathy of prematurity.N Engl J Med. 2011 Jun 16;364(24):2359; author reply 2361-2. doi: 10.1056/NEJMc1103460. N Engl J Med. 2011. PMID: 21675899 No abstract available.
References
-
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. - PubMed
-
- Katz X, Kychenthal A, Dorta P. Zone I retinopathy of prematurity. J AAPOS. 2000;4:373–6. - PubMed
-
- O’Keefe M, Lanigan B, Long VW. Outcomes of zone I retinopathy of prematurity. Acta Ophthalmol Scand. 2003;81:614–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
