The design, synthesis and pharmacological characterization of novel β₂-adrenoceptor antagonists
- PMID: 21323900
- PMCID: PMC3174413
- DOI: 10.1111/j.1476-5381.2011.01269.x
The design, synthesis and pharmacological characterization of novel β₂-adrenoceptor antagonists
Abstract
Background and purpose: Selective and potent antagonists for the β(2) -adrenoceptor are potentially interesting as experimental and clinical tools, and we sought to identify novel ligands with this pharmacology.
Experimental approach: A range of pharmacological assays was used to assess potency, affinity, selectivity (β(2) -adrenoceptor vs. β(1) -adrenoceptor) and efficacy.
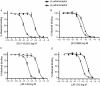
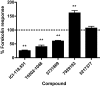
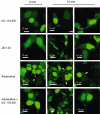
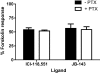
Key results: Ten novel compounds were identified but none had as high affinity as the prototypical β(2) -adrenoceptor blocker ICI-118,551, although one of the novel compounds was more selective for β(2) -adrenoceptors. Most of the ligands were inverse agonists for β(2) -adrenoceptor-cAMP signalling, although one (5217377) was a partial agonist and another a neutral antagonist (7929193). None of the ligands were efficacious with regard to β(2) -adrenoceptor-β-arrestin signalling. The (2S,3S) enantiomers were identified as the most active, although unusually the racemates were the most selective for the β(2) -adrenoceptors. This was taken as evidence for some unusual enantiospecific behaviour.
Conclusions and implications: In terms of improving on the pharmacology of the ligand ICI-118,551, one of the compounds was more selective (racemic JB-175), while one was a neutral antagonist (7929193), although none had as high an affinity. The results substantiate the notion that β-blockers do more than simply inhibit receptor activation, and differences between the ligands could provide useful tools to investigate receptor biology.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


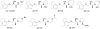
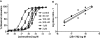


Similar articles
-
Beta 1- and beta 2-adrenoceptor antagonist activities of ICI-215001, a putative beta 3-adrenoceptor agonist.Br J Pharmacol. 1994 May;112(1):55-8. doi: 10.1111/j.1476-5381.1994.tb13028.x. Br J Pharmacol. 1994. PMID: 7913381 Free PMC article.
-
Comparison of the beta-adrenoceptor affinity and selectivity of cetamolol, atenolol, betaxolol, and ICI-118,551.J Cardiovasc Pharmacol. 1988 Aug;12(2):208-17. doi: 10.1097/00005344-198808000-00011. J Cardiovasc Pharmacol. 1988. PMID: 2459552
-
beta(2) and beta(3)-adrenoceptor inhibition of alpha(1)-adrenoceptor-stimulated Ca(2+) elevation in human cultured prostatic stromal cells.Eur J Pharmacol. 2007 Sep 10;570(1-3):18-26. doi: 10.1016/j.ejphar.2007.05.035. Epub 2007 Jun 5. Eur J Pharmacol. 2007. PMID: 17617401
-
Recruitment of β-arrestin 1 and 2 to the β2-adrenoceptor: analysis of 65 ligands.J Pharmacol Exp Ther. 2015 Nov;355(2):183-90. doi: 10.1124/jpet.115.227959. Epub 2015 Aug 25. J Pharmacol Exp Ther. 2015. PMID: 26306764 Free PMC article.
-
Adrenoceptors: Receptors, Ligands and Their Clinical Uses, Molecular Pharmacology and Assays.Handb Exp Pharmacol. 2024;285:55-145. doi: 10.1007/164_2024_713. Handb Exp Pharmacol. 2024. PMID: 38926158 Review.
Cited by
-
A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes.Cells. 2020 Aug 19;9(9):1923. doi: 10.3390/cells9091923. Cells. 2020. PMID: 32825009 Free PMC article.
-
Serotonin after β-Adrenoreceptors' Exposition: New Approaches for Personalized Data in Breast Cancer Cells.J Pers Med. 2021 Sep 25;11(10):954. doi: 10.3390/jpm11100954. J Pers Med. 2021. PMID: 34683096 Free PMC article.
-
Synthesis, docking study and β-adrenoceptor activity of some new oxime ether derivatives.Molecules. 2014 Mar 20;19(3):3417-35. doi: 10.3390/molecules19033417. Molecules. 2014. PMID: 24658567 Free PMC article.
-
Isoproterenol induces vascular oxidative stress and endothelial dysfunction via a Giα-coupled β2-adrenoceptor signaling pathway.PLoS One. 2014 Mar 12;9(3):e91877. doi: 10.1371/journal.pone.0091877. eCollection 2014. PLoS One. 2014. PMID: 24622771 Free PMC article.
-
Cell type-specific β2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity.J Biol Chem. 2012 May 11;287(20):16768-80. doi: 10.1074/jbc.M111.329912. Epub 2012 Mar 22. J Biol Chem. 2012. PMID: 22442147 Free PMC article.
References
-
- Alewijnse AE, Smit MJ, Rodriguez Pena MS, Verzijl D, Timmerman H, Leurs R. Modulation of forskolin-mediated adenylyl cyclase activation by constitutively active G(S)-coupled receptors. FEBS Lett. 1997;419:171–174. - PubMed
-
- Audet M, Bouvier M. Insights into signaling from the beta2-adrenergic receptor structure. Nat Chem Biol. 2008;4:397–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

