The cardioprotective efficacy of TVP1022 in a rat model of ischaemia/reperfusion
- PMID: 21323905
- PMCID: PMC3111678
- DOI: 10.1111/j.1476-5381.2011.01274.x
The cardioprotective efficacy of TVP1022 in a rat model of ischaemia/reperfusion
Abstract
Background and purpose: Because myocardial infarction is a major cause of morbidity and mortality worldwide, protecting the heart from the ischaemia and reperfusion (I/R) damage is the focus of intense research. Based on our in vitro findings showing that TVP1022 (the S-enantiomer of rasagiline, an anti-Parkinsonian drug) possesses cardioprotective effects, in the present study we investigated the hypothesis that TVP1022 can attenuate myocardial damage in an I/R model in rats.
Experimental approach: The model consisted of 30-min occlusion of the left anterior descending artery followed by 4 or 24 h reperfusion. In addition, we investigated the possible mechanisms of cardioprotection in H9c2 cells and neonatal rat ventricular myocytes (NRVM) exposed to oxidative stress induced by H(2) O(2) .
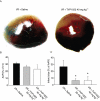
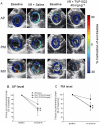
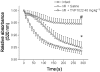
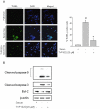
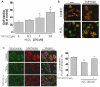
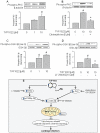
Key results: TVP1022 (20 and 40 mg·kg(-1) ) administered 5 min before reperfusion followed by an additional dose 4 h after reperfusion reduced the infarct size and attenuated the decline in ventricular function. TVP1022 also attenuated I/R-induced deterioration in cardiac mitochondrial integrity evaluated by mitochondrial swelling capacity. In vitro, using H9c2 cells and NRVM, TVP1022 attenuated both serum free- and H(2) O(2) -induced damage, preserved mitochondrial membrane potential and Bcl-2 levels, inhibited mitochondrial cytochrome c release and the increase in cleaved caspase 9 and 3 levels, and enhanced the phosphorylation of protein kinase C and glycogen synthase kinase-3β.
Conclusions and implications: TVP1022 provided cardioprotection in a model of myocardial infarction, and therefore should be considered as a novel adjunctive therapy for attenuating myocardial damage resulting from I/R injuries.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


Similar articles
-
TVP1022 and propargylamine protect neonatal rat ventricular myocytes against doxorubicin-induced and serum starvation-induced cardiotoxicity.J Cardiovasc Pharmacol. 2008 Sep;52(3):268-77. doi: 10.1097/FJC.0b013e3181862441. J Cardiovasc Pharmacol. 2008. PMID: 18806608
-
TVP1022: A Novel Cardioprotective Drug Attenuates Left Ventricular Remodeling After Ischemia/Reperfusion in Pigs.J Cardiovasc Pharmacol. 2015 Aug;66(2):214-22. doi: 10.1097/FJC.0000000000000267. J Cardiovasc Pharmacol. 2015. PMID: 25900266
-
Panax quinquefolium saponin attenuates cardiomyocyte apoptosis and opening of the mitochondrial permeability transition pore in a rat model of ischemia/reperfusion.Cell Physiol Biochem. 2014;34(4):1413-26. doi: 10.1159/000366347. Epub 2014 Oct 3. Cell Physiol Biochem. 2014. PMID: 25301366
-
Cardioprotective effect of melatonin against ischaemia/reperfusion damage.Front Biosci (Elite Ed). 2013 Jan 1;5(1):305-15. doi: 10.2741/e617. Front Biosci (Elite Ed). 2013. PMID: 23276991 Review.
-
Does inhibition of glycogen synthase kinase protect in mice?Circ Res. 2008 Aug 1;103(3):226-8. doi: 10.1161/CIRCRESAHA.108.181602. Circ Res. 2008. PMID: 18669927 Free PMC article. Review. No abstract available.
Cited by
-
Engineering of Reductive Aminases for Asymmetric Synthesis of Enantiopure Rasagiline.Front Bioeng Biotechnol. 2021 Dec 22;9:798147. doi: 10.3389/fbioe.2021.798147. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 35004654 Free PMC article.
-
I1 imidazoline receptor: novel potential cytoprotective target of TVP1022, the S-enantiomer of rasagiline.PLoS One. 2012;7(11):e47890. doi: 10.1371/journal.pone.0047890. Epub 2012 Nov 15. PLoS One. 2012. PMID: 23166584 Free PMC article.
-
The neuroprotective agent Rasagiline mesylate attenuates cardiac remodeling after experimental myocardial infarction.ESC Heart Fail. 2017 Aug;4(3):331-340. doi: 10.1002/ehf2.12140. Epub 2017 Mar 12. ESC Heart Fail. 2017. PMID: 28772050 Free PMC article.
-
The cardioprotective efficacy of TVP1022 against ischemia/reperfusion injury and cardiac remodeling in rats.Pharmacol Res Perspect. 2016 Nov 22;4(6):e00272. doi: 10.1002/prp2.272. eCollection 2016 Dec. Pharmacol Res Perspect. 2016. PMID: 28097005 Free PMC article.
-
7-Hydroxy Frullanolide Ameliorates Isoproterenol-Induced Myocardial Injury through Modification of iNOS and Nrf2 Genes.Biomedicines. 2023 Sep 6;11(9):2470. doi: 10.3390/biomedicines11092470. Biomedicines. 2023. PMID: 37760913 Free PMC article.
References
-
- Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. - PubMed
-
- Baines C, Zhang J, Wang G, Zheng Y, Xiu J, Cardwell E, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. - PubMed
-
- Bar-Am O, Weinreb O, Amit T, Youdim MB. Regulation of Bcl-2 family proteins, neurotrophic factors, and APP processing in the neurorescue activity of propargylamine. FASEB J. 2005;19:1899–1901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

