Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-α inhibitors
- PMID: 21324169
- PMCID: PMC3241369
- DOI: 10.1186/ar3249
Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-α inhibitors
Abstract
Introduction: The long-term treatment of rheumatoid arthritis (RA) most often involves a sequence of different therapies. The response to therapy, disease progression and detailed knowledge of the role of different therapies along treatment pathways are key aspects to help physicians identify the best treatment strategy. Thus, understanding the effectiveness of different therapeutic sequences is of particular importance in the evaluation of long-term RA treatment strategies. The objective of this study was to systematically review and quantitatively evaluate the relationship between the clinical response to biologic treatments and the number of previous treatments with tumor necrosis factor α (TNF-α) inhibitors.
Methods: A systematic search was undertaken to identify published, peer-reviewed articles that reported clinical outcomes of biologic treatment among RA patients with an inadequate response to TNF-α inhibitors. Data were systematically abstracted. Efficacy rates were estimated for groups of patients who differed in the number of prior TNF-α inhibitors used. End points included American College of Rheumatology (ACR)-, European League Against Rheumatism (EULAR)- and Disease Activity Score 28 (DAS28)-based response criteria.
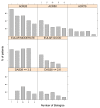
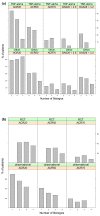
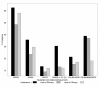
Results: The literature search identified 41 publications, of which 28 reported biologic treatment outcomes for RA patients with prior exposure to TNF-α inhibitors. Seven publications reported outcomes obtained in randomized clinical trials, while the remaining consisted of observational studies. The likelihood of responding to a subsequent biologic treatment decreased as the number of previous treatments with TNF-α inhibitors increased for six of the seven response criteria examined.
Conclusions: For patients with prior exposure to TNF-α inhibitors, the likelihood of response to subsequent treatment with biologic agents declines with the increasing number of previous treatments with TNF-α inhibitors.
Figures
References
-
- van Jaarsveld CH, Jacobs JW, van der Veen MJ, Blaauw AA, Kruize AA, Hofman DM, Brus HL, Albada-Kuipers GA, Heurkens AH, ter Borg EJ, Haanen HC, van Booma-Frankfort C, Schenk Y, Bijlsma JW. Aggressive treatment in early rheumatoid arthritis: a randomised controlled trial. On behalf of the Rheumatic Research Foundation Utrecht, The Netherlands. Ann Rheum Dis. 2000;59:468–477. doi: 10.1136/ard.59.6.468. - DOI - PMC - PubMed
-
- Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, Hazes JM. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446–451. doi: 10.1016/S0002-9343(01)00872-5. - DOI - PubMed
-
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O'Dell J, Turkiewicz AM, Furst DE. American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

