A randomized, double-blind, placebo-controlled trial of calcium acetate on serum phosphorus concentrations in patients with advanced non-dialysis-dependent chronic kidney disease
- PMID: 21324193
- PMCID: PMC3055808
- DOI: 10.1186/1471-2369-12-9
A randomized, double-blind, placebo-controlled trial of calcium acetate on serum phosphorus concentrations in patients with advanced non-dialysis-dependent chronic kidney disease
Abstract
Background: Hyperphosphatemia in patients with chronic kidney disease (CKD) contributes to secondary hyperparathyroidism, soft tissue calcification, and increased mortality risk. This trial was conducted to examine the efficacy and safety of calcium acetate in controlling serum phosphorus in pre-dialysis patients with CKD.
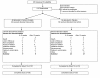
Methods: In this randomized, double-blind, placebo-controlled trial, 110 nondialyzed patients from 34 sites with estimated GFR < 30 mL/min/1.73 m² and serum phosphorus > 4.5 mg/dL were randomized to calcium acetate or placebo for 12 weeks. The dose of study drugs was titrated to achieve target serum phosphorus of 2.7-4.5 mg/dL. Serum phosphorus, calcium, iPTH, bicarbonate and serum albumin were measured at baseline and every 2 weeks for the 12 week study period. The primary efficacy endpoint was serum phosphorus at 12 weeks. Secondary endpoints were to measure serum calcium and intact parathyroid hormone (iPTH) levels.
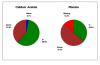
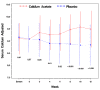
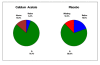
Results: At 12 weeks, serum phosphorus concentration was significantly lower in the calcium acetate group compared to the placebo group (4.4 ± 1.2 mg/dL vs. 5.1 ± 1.4 mg/dL; p = 0.04). The albumin-adjusted serum calcium concentration was significantly higher (9.5 ± 0.8 vs. 8.8 ± 0.8; p < 0.001) and iPTH was significantly lower in the calcium acetate group compared to placebo (150 ± 157 vs. 351 ± 292 pg/mL respectively; p < 0.001). At 12 weeks, the proportions of subjects who had hypocalcemia were 5.4% and 19.5% for the calcium acetate and the placebo groups, respectively, while the proportions of those with hypercalcemia were 13.5% and 0%, respectively. Adverse events did not differ between the treatment groups.
Conclusions: In CKD patients not yet on dialysis, calcium acetate was effective in reducing serum phosphorus and iPTH over a 12 week period.
Trial registration: www.clinicaltrials.gov NCT00211978.
Figures


References
-
- El Nahas AM, Bello AK. Chronic Kidney Disease: The global challenge. Lancet. 2005;365:331–340. - PubMed
-
- United States Renal Data System. 2000 Annual Data. Bethesda, MD, USA. 2000. http://www.USRDS.org
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

