Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients
- PMID: 21324198
- PMCID: PMC3221996
- DOI: 10.1186/cc10037
Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients
Abstract
Introduction: suPAR is the soluble form of the urokinase plasminogen activator receptor (uPAR), which is expressed in various immunologically active cells. High suPAR serum concentrations are suggested to reflect the activation of the immune system in circumstances of inflammation and infection, and have been associated with increased mortality in different populations of non-intensive care patients. In this study we sequentially analyzed suPAR serum concentrations within the first week of intensive care in a large cohort of well characterized intensive care unit (ICU) patients, in order to investigate potential regulatory mechanisms and evaluate the prognostic significance in critically ill patients.
Methods: A total of 273 patients (197 with sepsis, 76 without sepsis) were studied prospectively upon admission to the medical intensive care unit (ICU), on Day 3 and Day 7, and compared to 43 healthy controls. Clinical data, various laboratory parameters as well as investigational inflammatory cytokine profiles were assessed. Patients were followed for approximately one year.
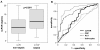
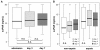
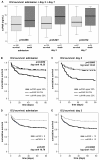
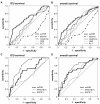
Results: Upon admission to the ICU suPAR serum concentrations were elevated in critically ill patients as compared with healthy controls. In sepsis patients suPAR levels were higher than in non-sepsis patients (with or without systemic inflammatory response syndrome (SIRS)). During the first week after admission to the ICU serum suPAR concentrations remained stably elevated. suPAR serum concentrations measured upon admission were closely and independently correlated to various laboratory parameters, specifically biomarkers of inflammation (tumor necrosis factor (TNF), C-reactive protein (CRP)), hepatic and renal dysfunction. High suPAR levels at admission and at Day 3 were a strong independent predictor for both ICU and long-term mortality in critically ill patients.
Conclusions: In sepsis and non-sepsis patients suPAR serum concentrations are increased upon admission to the ICU, likely reflecting the activation state of the immune system, and remain stably elevated in the initial course of treatment. Low suPAR levels are a positive predictor of ICU- and overall survival in critically ill patients, including sepsis and non-sepsis patients. Aside from its value as a promising new prognostic biomarker, both experimental and clinical studies are required in order to understand the specific effects and regulatory mechanisms of suPAR in SIRS and sepsis, and may reveal new therapeutic options.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

