Genetic monitoring detects an overlooked cryptic species and reveals the diversity and distribution of three invasive Rattus congeners in South Africa
- PMID: 21324204
- PMCID: PMC3055845
- DOI: 10.1186/1471-2156-12-26
Genetic monitoring detects an overlooked cryptic species and reveals the diversity and distribution of three invasive Rattus congeners in South Africa
Abstract
Background: South Africa's long and extensive trade activity has ensured ample opportunities for exotic species introduction. Whereas the rich biodiversity of endemic southern African fauna has been the focus of many studies, invasive vertebrates are generally overlooked despite potential impacts on biodiversity, health and agriculture. Genetic monitoring of commensal rodents in South Africa which uncovered the presence of Rattus tanezumi, a South-East Asian endemic not previously known to occur in Africa, provided the impetus for expanded studies on all invasive Rattus species present.
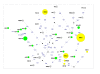
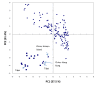
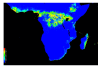
Results: To this end, intensified sampling at 28 South African localities and at one site in Swaziland, identified 149 Rattus specimens. Cytochrome b gene sequencing revealed the presence of two R. tanezumi, seven Rattus rattus and five Rattus norvegicus haplotypes in south Africa. Phylogenetic results were consistent with a single, recent R. tanezumi introduction and indicated that R. norvegicus and R. rattus probably became established following at least two and three independent introductions, respectively. Intra- and inter-specific diversity was highest in informal human settlements, with all three species occurring at a single metropolitan township site. Rattus norvegicus and R. rattus each occurred sympatrically with Rattus tanezumi at one and five sites, respectively. Karyotyping of selected R. rattus and R. tanezumi individuals identified diploid numbers consistent with those reported previously for these cryptic species. Ordination of bioclimatic variables and MaxEnt ecological niche modelling confirmed that the bioclimatic niche occupied by R. tanezumi in south Africa was distinct from that occupied in its naturalised range in south-east Asia suggesting that factors other than climate may influence the distribution of this species.
Conclusions: This study has highlighted the value of genetic typing for detecting cryptic invasive species, providing historical insights into introductions and for directing future sampling. The apparent ease with which a cryptic species can become established signals the need for broader implementation of genetic monitoring programmes. In addition to providing baseline data and potentially identifying high-risk introduction routes, the predictive power of ecological niche modelling is enhanced when species records are genetically verified.
Figures





References
-
- Aplin KP, Chesser T, ten Have J. In: Rats, Mice and People: Rodent Biology and Management. Singleton GR, Hinds LA, Krebs CJ, Spratt DM, editor. Canberra: The Australian Centre for International Agricultural Research (ACIAR); 2003. Evolutionary biology of the genus Rattus: profile of an archetypal rodent pest; pp. 487–498.
-
- Matisoo-Smith E, Robins J. Mitochondrial DNA evidence for the spread of Pacific rats through Oceania. Biological Invasions. 2009;12:1521–1527. doi: 10.1007/s10530-008-9404-1. - DOI
-
- Tollenaere C, Brouat C, Duplantier J-M, Rahalison L, Rahelinirina S, Pascal M, Moné H, Mouahid G, Leirs H, Cosson J-F. Phylogeography of the introduced species Rattus rattus in the western Indian Ocean, with special emphasis on the colonization history of Madagascar. J Biogeogr. 2010;12:398–410. doi: 10.1111/j.1365-2699.2009.02228.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

