The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle
- PMID: 21324402
- PMCID: PMC3057410
- DOI: 10.1016/j.biomaterials.2011.01.062
The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle
Abstract
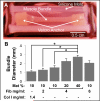
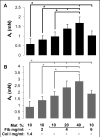
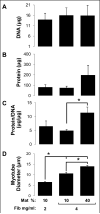
One of the obstacles to the potential clinical utility of bioengineered skeletal muscle is its limited force generation capacity. Since engineered muscle, unlike most native muscle tissue, is composed of relatively short myofibers, we hypothesized that, its force production and transmission would be profoundly influenced by cell-matrix interactions. To test this hypothesis, we systematically varied the matrix protein type (collagen I/fibrin/Matrigel) and concentration in engineered, hydrogel-based neonatal rat skeletal muscle bundles and assessed the resulting tissue structure, generation of contractile force, and intracellular Ca(2+) handling. After two weeks of culture, the muscle bundles consisted of highly aligned and cross-striated myofibers and exhibited standard force-length and force-frequency relationships achieving tetanus at 40 Hz. The use of 2 mg/ml fibrin (control) yielded isometric tetanus amplitude of 1.4 ± 0.3 mN as compared to 0.9 ± 0.4 mN measured in collagen I-based bundles. Higher fibrin and Matrigel concentrations synergistically yielded further increase in active force generation to 2.8 ± 0.5 mN without significantly affecting passive mechanical properties, tetanus-to-twitch ratio, and twitch kinetics. Optimized matrix composition yielded significant cellular hypertrophy (protein/DNA ratio = 11.4 ± 4.1 vs. 6.5 ± 1.9 μg/μg in control) and a prolonged Ca(2+) transient half-width (Ca(50) = 232.8 ± 33.3 vs. 101.7 ± 19.8 ms). The use of growth-factor-reduced Matrigel, instead of standard Matrigel did not alter the obtained results suggesting enhanced cell-matrix interactions rather than growth factor supplementation as an underlying cause for the measured increase in contractile force. In summary, biomaterial-based manipulation of cell-matrix interactions represents an important target for improving contractile force generation in engineered skeletal muscle.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

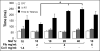
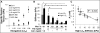

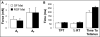
References
-
- Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001;12:64–75. - PubMed
-
- Vilquin JT. Myoblast transplantation: clinical trials and perspectives. Mini-review. Acta Myol. 2005;24:119–27. - PubMed
-
- Liao H, Zhou GQ. Development and progress of engineering of skeletal muscle tissue. Tissue Eng Part B Rev. 2009;15:319–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

