Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial
- PMID: 21324520
- PMCID: PMC4852146
- DOI: 10.1016/S0140-6736(10)62145-9
Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial
Abstract
Background: Daily inhaled corticosteroids are an effective treatment for mild persistent asthma, but some children have exacerbations even with good day-to-day control, and many discontinue treatment after becoming asymptomatic. We assessed the effectiveness of an inhaled corticosteroid (beclomethasone dipropionate) used as rescue treatment.
Methods: In this 44-week, randomised, double-blind, placebo-controlled trial we enrolled children and adolescents with mild persistent asthma aged 5-18 years from five clinical centres in the USA. A computer-generated randomisation sequence, stratified by clinical centre and age group, was used to randomly assign participants to one of four treatment groups: twice daily beclomethasone with beclomethasone plus albuterol as rescue (combined group); twice daily beclomethasone with placebo plus albuterol as rescue (daily beclomethasone group); twice daily placebo with beclomethasone plus albuterol as rescue (rescue beclomethasone group); and twice daily placebo with placebo plus albuterol as rescue (placebo group). Twice daily beclomethasone treatment was one puff of beclomethasone (40 μg per puff) or placebo given in the morning and evening. Rescue beclomethasone treatment was two puffs of beclomethasone or placebo for each two puffs of albuterol (180 μg) needed for symptom relief. The primary outcome was time to first exacerbation that required oral corticosteroids. A secondary outcome measured linear growth. Analysis was by intention to treat. This study is registered with clinicaltrials.gov, number NCT00394329.
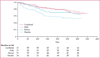
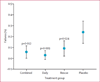
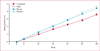
Results: 843 children and adolescents were enrolled into this trial, of whom 288 were assigned to one of four treatment groups; combined (n=71), daily beclomethasone (n=72), rescue beclomethasone (n=71), and placebo (n=74)-555 individuals were excluded during the run-in, according to predefined criteria. Compared with the placebo group (49%, 95% CI 37-61), the frequency of exacerbations was lower in the daily (28%, 18-40, p=0·03), combined (31%, 21-43, p=0·07), and rescue (35%, 24-47, p=0·07) groups. Frequency of treatment failure was 23% (95% CI 14-43) in the placebo group, compared with 5·6% (1·6-14) in the combined (p=0·012), 2·8% (0-10) in the daily (p=0·009), and 8·5% (2-15) in the rescue (p=0·024) groups. Compared with the placebo group, linear growth was 1·1 cm (SD 0·3) less in the combined and daily arms (p<0·0001), but not the rescue group (p=0·26). Only two individuals had severe adverse events; one in the daily beclomethasone group had viral meningitis and one in the combined group had bronchitis.
Interpretation: Children with mild persistent asthma should not be treated with rescue albuterol alone and the most effective treatment to prevent exacerbations is daily inhaled corticosteroids. Inhaled corticosteroids as rescue medication with albuterol might be an effective step-down strategy for children with well controlled, mild asthma because it is more effective at reducing exacerbations than is use of rescue albuterol alone. Use of daily inhaled corticosteroid treatment and related side-effects such as growth impairment can therefore be avoided.
Funding: National Heart, Lung and Blood Institute.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All other authors declare that they have no conflicts of interest.
Figures

Comment in
-
New insights into the treatment of persistent asthma.Lancet. 2011 Feb 19;377(9766):614-6. doi: 10.1016/S0140-6736(10)62313-6. Epub 2011 Feb 14. Lancet. 2011. PMID: 21324521 No abstract available.
-
In children and adolescents with mild persistent asthma, daily beclomethasone reduces treatment failure compared with rescue beclomethasone plus albuterol.Evid Based Med. 2011 Dec;16(6):183-4. doi: 10.1136/ebm1411. Epub 2011 May 11. Evid Based Med. 2011. PMID: 21561924 No abstract available.
-
Treatment of mild persistent asthma in children.Lancet. 2011 May 21;377(9779):1743; author reply 1744. doi: 10.1016/S0140-6736(11)60724-1. Lancet. 2011. PMID: 21601698 No abstract available.
-
Treatment of mild persistent asthma in children.Lancet. 2011 May 21;377(9779):1743; author reply 1744. doi: 10.1016/S0140-6736(11)60723-X. Lancet. 2011. PMID: 21601699 No abstract available.
-
Treatment of mild persistent asthma in children.Lancet. 2011 May 21;377(9779):1743-4; author reply 1744. doi: 10.1016/S0140-6736(11)60725-3. Lancet. 2011. PMID: 21601700 No abstract available.
-
Inhaled corticosteroids are beneficial in treating asthma exacerbations in children with mild persistent asthma.J Pediatr. 2011 Sep;159(3):513-4. doi: 10.1016/j.jpeds.2011.07.005. J Pediatr. 2011. PMID: 21846526 No abstract available.
References
-
- National Asthma Education Prevention Program Coordinating Committee Expert Panel. [accessed 17 Jan, 2011];Guidelines for the diagnosis and management of asthma. 2007 http://www.nhlbi.nih.gov/guidelines/asthma/
-
- Global Initiative for Asthma (GINA) [accessed Jan 17, 2011];Global strategy for asthma management and prevention. 2009 http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=1561.
-
- Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008;13:559–563. - PubMed
-
- Sorkness CA, Lemanske RF, Jr, Mauger DT, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. - PubMed
-
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

