Quantitative imaging test approval and biomarker qualification: interrelated but distinct activities
- PMID: 21325035
- PMCID: PMC5410955
- DOI: 10.1148/radiol.10100800
Quantitative imaging test approval and biomarker qualification: interrelated but distinct activities
Abstract
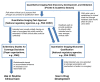
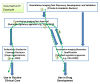
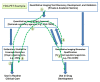
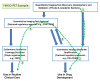
Quantitative imaging biomarkers could speed the development of new treatments for unmet medical needs and improve routine clinical care. However, it is not clear how the various regulatory and nonregulatory (eg, reimbursement) processes (often referred to as pathways) relate, nor is it clear which data need to be collected to support these different pathways most efficiently, given the time- and cost-intensive nature of doing so. The purpose of this article is to describe current thinking regarding these pathways emerging from diverse stakeholders interested and active in the definition, validation, and qualification of quantitative imaging biomarkers and to propose processes to facilitate the development and use of quantitative imaging biomarkers. A flexible framework is described that may be adapted for each imaging application, providing mechanisms that can be used to develop, assess, and evaluate relevant biomarkers. From this framework, processes can be mapped that would be applicable to both imaging product development and to quantitative imaging biomarker development aimed at increasing the effectiveness and availability of quantitative imaging.
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.10100800/-/DC1.
RSNA, 2011
Figures

References
-
- Woodcock J, Woosley R. The FDA Critical Path Initiative and its influence on new drug development. Annu Rev Med 2008;59:1–12. - PubMed
-
- Challenges and Opportunities Report—March 2004. Innovation or stagnation: challenge and opportunity on the critical path to new medical products. U.S. Food and Drug Administration. http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/.... Last updated July 20, 2010. Accessed January 5, 2010.
-
- The Innovative Medicines Initiative Web site. http://www.imi-europe.org/. Accessed March 17, 2010.
-
- Hunter AJ. The Innovative Medicines Initiative: a pre-competitive initiative to enhance the biomedical science base of Europe to expedite the development of new medicines for patients. Drug Discov Today 2008;13(9-10):371–373. - PubMed
-
- Biomarkers Consortium Web site. http://www.biomarkersconsortium.org/. Accessed March 17, 2010.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

