Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth
- PMID: 21325052
- PMCID: PMC3054028
- DOI: 10.1073/pnas.1014769108
Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth
Abstract
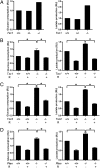
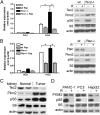
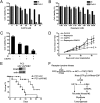
Although aerobic glycolysis (the Warburg effect) is a hallmark of cancer, key questions, including when, how, and why cancer cells become highly glycolytic, remain less clear. For a largely unknown regulatory mechanism, a rate-limiting glycolytic enzyme pyruvate kinase M2 (PKM2) isoform is exclusively expressed in embryonic, proliferating, and tumor cells, and plays an essential role in tumor metabolism and growth. Because the receptor tyrosine kinase/PI3K/AKT/mammalian target of rapamycin (RTK/PI3K/AKT/mTOR) signaling cascade is a frequently altered pathway in cancer, we explored its potential role in cancer metabolism. We identified mTOR as a central activator of the Warburg effect by inducing PKM2 and other glycolytic enzymes under normoxic conditions. PKM2 level was augmented in mouse kidney tumors due to deficiency of tuberous sclerosis complex 2 and consequent mTOR activation, and was reduced in human cancer cells by mTOR suppression. mTOR up-regulation of PKM2 expression was through hypoxia-inducible factor 1α (HIF1α)-mediated transcription activation, and c-Myc-heterogeneous nuclear ribonucleoproteins (hnRNPs)-dependent regulation of PKM2 gene splicing. Disruption of PKM2 suppressed oncogenic mTOR-mediated tumorigenesis. Unlike normal cells, mTOR hyperactive cells were more sensitive to inhibition of mTOR or glycolysis. Dual suppression of mTOR and glycolysis synergistically blunted the proliferation and tumor development of mTOR hyperactive cells. Even though aerobic glycolysis is not required for breach of senescence for immortalization and transformation, the frequently deregulated mTOR signaling during multistep oncogenic processes could contribute to the development of the Warburg effect in many cancers. Components of the mTOR/HIF1α/Myc-hnRNPs/PKM2 glycolysis signaling network could be targeted for the treatment of cancer caused by an aberrant RTK/PI3K/AKT/mTOR signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: Dawn of a new era? Sci STKE. 2007;2007:pe14. - PubMed
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Yoo YG, Hayashi M, Christensen J, Huang LE. An essential role of the HIF-1alpha-c-Myc axis in malignant progression. Ann N Y Acad Sci. 2009;1177:198–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

