Two ground state isoforms and a chromophore D-ring photoflip triggering extensive intramolecular changes in a canonical phytochrome
- PMID: 21325055
- PMCID: PMC3054036
- DOI: 10.1073/pnas.1013377108
Two ground state isoforms and a chromophore D-ring photoflip triggering extensive intramolecular changes in a canonical phytochrome
Abstract
Phytochrome photoreceptors mediate light responses in plants and in many microorganisms. Here we report studies using (1)H-(13)C magic-angle spinning NMR spectroscopy of the sensor module of cyanobacterial phytochrome Cph1. Two isoforms of the red-light absorbing Pr ground state are identified. Conclusive evidence that photoisomerization occurs at the C15-methine bridge leading to a β-facial disposition of the ring D is presented. In the far-red-light absorbing Pfr state, strong hydrogen-bonding interactions of the D-ring carbonyl group to Tyr-263 and of N24 to Asp-207 hold the chromophore in a tensed conformation. Signaling is triggered when Asp-207 is released from its salt bridge to Arg-472, probably inducing conformational changes in the tongue region. A second signal route is initiated by partner swapping of the B-ring propionate between Arg-254 and Arg-222.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 interfacial correlations during Pr → Pfr photoconversion. All 1H contacts of the chromophore are summarized in
interfacial correlations during Pr → Pfr photoconversion. All 1H contacts of the chromophore are summarized in 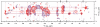
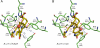

References
-
- Smith H. Phytochromes and light signal perception by plants—an emerging synthesis. Nature. 2000;407:585–591. - PubMed
-
- Hughes J, et al. A prokaryotic phytochrome. Nature. 1997;386:663. - PubMed
-
- Yeh K-C, Wu S-H, Murphy JT, Lagarias JC. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. - PubMed
-
- Davis SJ, Vener AV, Vierstra RD. Bacteriophytochromes: Phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science. 1999;286:2517–2520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

