Natural loss-of-function mutation of myeloid differentiation protein 88 disrupts its ability to form Myddosomes
- PMID: 21325272
- PMCID: PMC3064238
- DOI: 10.1074/jbc.M110.199653
Natural loss-of-function mutation of myeloid differentiation protein 88 disrupts its ability to form Myddosomes
Abstract
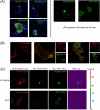
Myeloid differentiation protein 88 (MyD88) is a key signaling adapter in Toll-like receptor (TLR) signaling. MyD88 is also one of the most polymorphic adapter proteins. We screened the reported nonsynonymous coding mutations in MyD88 to identify variants with altered function. In reporter assays, a death domain variant, S34Y, was found to be inactive. Importantly, in reconstituted macrophage-like cell lines derived from knock-out mice, MyD88 S34Y was severely compromised in its ability to respond to all MyD88-dependent TLR ligands. Unlike wild-type MyD88, S34Y is unable to form distinct foci in the cells but is present diffused in the cytoplasm. We observed that IRAK4 co-localizes with MyD88 in these aggregates, and thus these foci appear to be "Myddosomes." The MyD88 S34Y loss-of-function mutant demonstrates how proper cellular localization of MyD88 to the Myddosome is a feature required for MyD88 function.
Figures
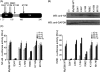
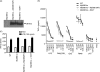




References
-
- Kawai T., Akira S. (2010) Nat. Immunol. 11, 373–384 - PubMed
-
- Akahoshi M., Nakashima H., Sadanaga A., Miyake K., Obara K., Tamari M., Hirota T., Matsuda A., Shirakawa T. (2008) Lupus 17, 568–574 - PubMed
-
- De Jager P. L., Richardson A., Vyse T. J., Rioux J. D. (2006) Arthritis Rheum. 54, 1279–1282 - PubMed
-
- Hamann L., Glaeser C., Hamprecht A., Gross M., Gomma A., Schumann R. R. (2006) Clin. Chim. Acta 364, 303–307 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

