Increased IGF-1 in muscle modulates the phenotype of severe SMA mice
- PMID: 21325354
- PMCID: PMC3071675
- DOI: 10.1093/hmg/ddr067
Increased IGF-1 in muscle modulates the phenotype of severe SMA mice
Abstract
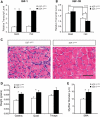
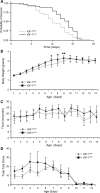
Spinal muscular atrophy (SMA) is an inherited motor neuron disease caused by the mutation of the survival motor neuron 1 (SMN1) gene and deficiency of the SMN protein. Severe SMA mice have abnormal motor function and small, immature myofibers early in development suggesting that SMN protein deficiency results in retarded muscle growth. Insulin-like growth factor 1 (IGF-1) stimulates myoblast proliferation, induces myogenic differentiation and generates myocyte hypertrophy in vitro and in vivo. We hypothesized that increased expression of IGF-1 specifically in skeletal muscle would attenuate disease features of SMAΔ7 mice. SMAΔ7 mice overexpressing a local isoform of IGF-1 (mIGF-1) in muscle showed enlarged myofibers and a 40% increase in median survival compared with mIGF-1-negative SMA littermates (median survival = 14 versus 10 days, respectively, log-rank P = 0.025). Surprisingly, this was not associated with a significant improvement in motor behavior. Treatment of both mIGF-1(NEG) and mIGF-1(POS) SMA mice with the histone deacetylase inhibitor, trichostatin A (TSA), resulted in a further extension of survival and improved motor behavior, but the combination of mIGF-1 and TSA treatment was not synergistic. These results show that increased mIGF-1 expression restricted to muscle can modulate the phenotype of SMA mice indicating that therapeutics targeted to muscle alone should not be discounted as potential disease-modifying therapies in SMA. IGF-1 may warrant further investigation in mild SMA animal models and perhaps SMA patients.
Figures


References
-
- Dubowitz V. Muscle Disorders in Childhood. Philadelphia: WB Saunders; 1995.
-
- Crawford T.O., Pardo C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. doi:10.1006/nbdi.1996.0010. - DOI - PubMed
-
- Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi:10.1016/0092-8674(95)90460-3. - DOI - PubMed
-
- Fidzianska A., Goebel H.H., Warlo I. Acute infantile spinal muscular atrophy. Muscle apoptosis as a proposed pathogenetic mechanism. Brain. 1990;113:433–445. doi:10.1093/brain/113.2.433. - DOI - PubMed
-
- Stevens L., Bastide B., Maurage C.A., Dupont E., Montel V., Cieniewski-Bernard C., Cuisset J.M., Vallee L., Mounier Y. Childhood spinal muscular atrophy induces alterations in contractile and regulatory protein isoform expressions. Neuropathol. Appl. Neurobiol. 2008;34:659–670. doi:10.1111/j.1365-2990.2008.00950.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

