Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species
- PMID: 21325418
- PMCID: PMC3126247
- DOI: 10.1128/JVI.02395-10
Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species
Abstract
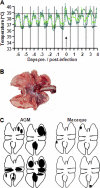
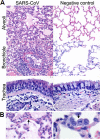
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), caused by influenza A virus H5N1 and severe acute respiratory syndrome coronavirus (SARS-CoV), supposedly depend on activation of the oxidative-stress machinery that is coupled with innate immunity, resulting in a strong proinflammatory host response. Inflammatory cytokines, such as interleukin 1β (IL-1β), IL-8, and IL-6, play a major role in mediating and amplifying ALI/ARDS by stimulating chemotaxis and activation of neutrophils. To obtain further insight into the pathogenesis of SARS-CoV-associated ALI, we compared SARS-CoV infections in two different nonhuman primate species, cynomolgus macaques and African green monkeys. Viral titers in the upper and lower respiratory tract were not significantly different in SARS-CoV-infected macaques and African green monkeys. Inflammatory cytokines that play a major role in mediating and amplifying ALI/ARDS or have neutrophil chemoattractant activity, such as IL-6, IL-8, CXCL1, and CXCL2, were, however, induced only in macaques. In contrast, other proinflammatory cytokines and chemokines, including osteopontin and CCL3, were upregulated in the lungs of African green monkeys to a significantly greater extent than in macaques. Because African green monkeys developed more severe ALI than macaques, with hyaline membrane formation, some of these differentially expressed proinflammatory genes may be critically involved in development of the observed pathological changes. Induction of distinct proinflammatory genes after SARS-CoV infection in different nonhuman primate species needs to be taken into account when analyzing outcomes of intervention strategies in these species.
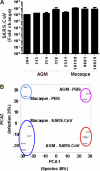
Figures



References
-
- Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Soc. B 57:289–300
-
- Berman J. S., et al. 2004. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L1311–L1318 - PubMed
-
- Chabas D., et al. 2001. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294:1731–1735 - PubMed
-
- Chen M., et al. 2009. Regulatory effects of IFN-beta on production of osteopontin and IL-17 by CD4+ T cells in MS. Eur. J. Immunol. 39:2525–2536 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

