Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer
- PMID: 21325445
- PMCID: PMC3179412
- DOI: 10.1093/annonc/mdq735
Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer
Abstract
Background: The International Breast Cancer Study Group Trial VIII compared long-term efficacy of endocrine therapy (goserelin), chemotherapy [cyclophosphamide, methotrexate and fluorouracil (CMF)], and chemoendocrine therapy (CMF followed by goserelin) for pre/perimenopausal women with lymph-node-negative breast cancer.
Patients and methods: From 1990 to 1999, 1063 patients were randomized to receive (i) goserelin for 24 months (n = 346), (ii) six courses of 'classical' CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy (n = 360), or (iii) six courses of CMF plus 18 months goserelin (CMF→ goserelin; n = 357). Tumors were classified as estrogen receptor (ER) negative (19%), ER positive (80%), or ER unknown (1%); 19% of patients were younger than 40. Median follow-up was 12.1 years.
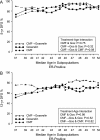
Results: For the ER-positive cohort, sequential therapy provided a statistically significant benefit in disease-free survival (DFS) (12-year DFS = 77%) compared with CMF alone (69%) and goserelin alone (68%) (P = 0.04 for each comparison), due largely to the effect in younger patients. Patients with ER-negative tumors whose treatment included CMF had similar DFS (12-year DFS CMF = 67%; 12-year DFS CMF→ goserelin = 69%) compared with goserelin alone (12-year DFS = 61%, P= NS).
Conclusions: For pre/perimenopausal women with lymph-node-negative ER-positive breast cancer, CMF followed by goserelin improved DFS in comparison with either modality alone. The improvement was the most pronounced in those aged below 40, suggesting an endocrine effect of prolonged CMF-induced amenorrhea.
Figures



References
-
- Early Breast Cancer Clinical Trialists' Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Viale G, Regan MM, Maiorano E, et al. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors–International Breast Cancer Study Group. J Clin Oncol. 2008;26:1404–1410. - PubMed
-
- Colozza M, Azambuja E, Cardoso F, et al. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–1739. - PubMed

