Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant
- PMID: 21325519
- PMCID: PMC6623704
- DOI: 10.1523/JNEUROSCI.5245-10.2011
Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant
Abstract
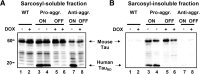
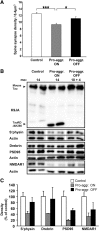
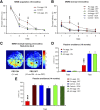
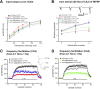
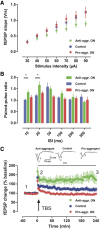
This report describes the behavioral and electrophysiological analysis of regulatable transgenic mice expressing mutant repeat domains of human Tau (Tau(RD)). Mice were generated to express Tau(RD) in two forms, differing in their propensity for β-structure and thus in their tendency for aggregation ("pro-aggregant" or "anti-aggregant") (Mocanu et al., 2008). Only pro-aggregant mice show pronounced changes typical for Tau pathology in Alzheimer's disease (aggregation, missorting, hyperphosphorylation, synaptic and neuronal loss), indicating that the β-propensity and hence the ability to aggregate is a key factor in the disease. We now tested the mice with regard to neuromotor parameters, behavior, learning and memory, and synaptic plasticity and correlated this with histological and biochemical parameters in different stages of switching Tau(RD) on or off. The mice are normal in neuromotor tests. However, pro-aggregant Tau(RD) mice are strongly impaired in memory and show pronounced loss of long-term potentiation (LTP), suggesting that Tau aggregation specifically perturbs these brain functions. Remarkably, when the expression of human pro-aggregant Tau(RD) is switched on for ∼ 10 months and off for ∼ 4 months, memory and LTP recover, whereas aggregates decrease moderately and change their composition from mixed human plus mouse Tau to mouse Tau only. Neuronal loss persists, but synapses are partially rescued. This argues that continuous presence of amyloidogenic pro-aggregant Tau(RD) constitutes the main toxic insult for memory and LTP, rather than the aggregates as such.
Figures





Similar articles
-
Reversibility of Tau-related cognitive defects in a regulatable FTD mouse model.J Mol Neurosci. 2011 Nov;45(3):432-7. doi: 10.1007/s12031-011-9604-5. Epub 2011 Aug 6. J Mol Neurosci. 2011. PMID: 21822709
-
Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau.Acta Neuropathol. 2012 Jun;123(6):787-805. doi: 10.1007/s00401-012-0987-3. Epub 2012 Apr 25. Acta Neuropathol. 2012. PMID: 22532069 Free PMC article.
-
Functional networks are impaired by elevated tau-protein but reversible in a regulatable Alzheimer's disease mouse model.Mol Neurodegener. 2019 Mar 27;14(1):13. doi: 10.1186/s13024-019-0316-6. Mol Neurodegener. 2019. PMID: 30917861 Free PMC article.
-
Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of Tau pathology.FEBS J. 2013 Sep;280(18):4371-81. doi: 10.1111/febs.12250. Epub 2013 Apr 22. FEBS J. 2013. PMID: 23517246 Review.
-
Acetylated tau in Alzheimer's disease: An instigator of synaptic dysfunction underlying memory loss: Increased levels of acetylated tau blocks the postsynaptic signaling required for plasticity and promotes memory deficits associated with tauopathy.Bioessays. 2017 Apr;39(4):10.1002/bies.201600224. doi: 10.1002/bies.201600224. Epub 2017 Jan 13. Bioessays. 2017. PMID: 28083916 Free PMC article. Review.
Cited by
-
Reduction of mutant ataxin-7 expression restores motor function and prevents cerebellar synaptic reorganization in a conditional mouse model of SCA7.Hum Mol Genet. 2013 Mar 1;22(5):890-903. doi: 10.1093/hmg/dds495. Epub 2012 Nov 29. Hum Mol Genet. 2013. PMID: 23197655 Free PMC article.
-
Protein tau: prime cause of synaptic and neuronal degeneration in Alzheimer's disease.Int J Alzheimers Dis. 2012;2012:251426. doi: 10.1155/2012/251426. Epub 2012 Jun 8. Int J Alzheimers Dis. 2012. PMID: 22720188 Free PMC article.
-
Synapses and Alzheimer's disease.Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a005777. doi: 10.1101/cshperspect.a005777. Cold Spring Harb Perspect Biol. 2012. PMID: 22491782 Free PMC article. Review.
-
Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss.Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):E2518-27. doi: 10.1073/pnas.1306832110. Epub 2013 Jun 17. Proc Natl Acad Sci U S A. 2013. PMID: 23776240 Free PMC article.
-
Formation and propagation of tau oligomeric seeds.Front Neurol. 2013 Jul 17;4:93. doi: 10.3389/fneur.2013.00093. eCollection 2013. Front Neurol. 2013. PMID: 23882255 Free PMC article.
References
-
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. - PMC - PubMed
-
- Arendash GW, Lewis J, Leighty R, McGowan E, Cracchiolo JR, Hutton M, Garcia MF. Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer's disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Res. 2004;1012:29–34. - PubMed
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
-
- Barghorn S, Zheng-Fischhöfer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39:11714–11721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
