The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse
- PMID: 21326213
- PMCID: PMC3094108
- DOI: 10.1038/emboj.2011.25
The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse
Abstract
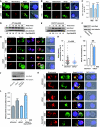
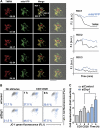
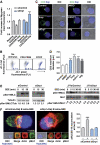
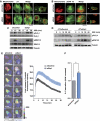
During antigen-specific T-cell activation, mitochondria mobilize towards the vicinity of the immune synapse. We show here that the mitochondrial fission factor dynamin-related protein 1 (Drp1) docks at mitochondria, regulating their positioning and activity near the actin-rich ring of the peripheral supramolecular activation cluster (pSMAC) of the immune synapse. Mitochondrial redistribution in response to T-cell receptor engagement was abolished by Drp1 silencing, expression of the phosphomimetic mutant Drp1S637D and the Drp1-specific inhibitor mdivi-1. Moreover, Drp1 knockdown enhanced mitochondrial depolarization and T-cell receptor signal strength, but decreased myosin phosphorylation, ATP production and T-cell receptor assembly at the central supramolecular activation cluster (cSMAC). Our results indicate that Drp1-dependent mitochondrial positioning and activity controls T-cell activation by fuelling central supramolecular activation cluster assembly at the immune synapse.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Comment in
-
Immune synapses: mitochondrial morphology matters.EMBO J. 2011 Apr 6;30(7):1187-9. doi: 10.1038/emboj.2011.72. EMBO J. 2011. PMID: 21468096 Free PMC article. Review.
References
-
- Calabia-Linares C, Robles-Valero J, De La Fuente H, Perez-Martínez M, Martin-Cofreces NB, Alfonso-Pérez M, Gutierrez-Vázquez C, María Mittelbrunn M, Ibiza SU-O FR, Aguado-Ballano C, Sánchez-Sorzano CO, Sánchez-Madrid F, Veiga E (2011) Endosomal clathrin drives actin accumulation at the immunological synapse. J Cell Sci (doi:10.1242/jcs078832) - PubMed
-
- Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD (2004) LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 20: 589–599 - PubMed
-
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14: 193–204 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

