A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours
- PMID: 21326243
- PMCID: PMC3048218
- DOI: 10.1038/bjc.2011.8
A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours
Abstract
Background: Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) is essential in cellular processing of DNA damage via the base excision repair pathway (BER). The PARP inhibition can be directly cytotoxic to tumour cells and augments the anti-tumour effects of DNA-damaging agents. This study evaluated the optimally tolerated dose of olaparib (4-(3--4-fluorophenyl) methyl-1(2H)-one; AZD2281, KU0059436), a potent PARP inhibitor, with dacarbazine and assessed safety, toxicity, clinical pharmacokinetics and efficacy of combination treatment.
Patients and methods: Patients with advanced cancer received olaparib (20-200 mg PO) on days 1-7 with dacarbazine (600-800 mg m(-2) IV) on day 1 (cycle 2, day 2) of a 21-day cycle. An expansion cohort of chemonaive melanoma patients was treated at an optimally tolerated dose. The BER enzyme, methylpurine-DNA glycosylase and its substrate 7-methylguanine were quantified in peripheral blood mononuclear cells.
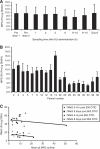
Results: The optimal combination to proceed to phase II was defined as 100 mg bd olaparib with 600 mg m(-2) dacarbazine. Dose-limiting toxicities were neutropaenia and thrombocytopaenia. There were two partial responses, both in patients with melanoma.
Conclusion: This study defined a tolerable dose of olaparib in combination with dacarbazine, but there were no responses in chemonaive melanoma patients, demonstrating no clinical advantage over single-agent dacarbazine at these doses.
Figures
References
-
- Bajetta E, Di Leo A, Zampino MG, Sertoli MR, Comella G, Barduagni M, Giannotti B, Queirolo P, Tribbia G, Bernengo MG (1994) Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. J Clin Oncol 12: 806–811 - PubMed
-
- Chalmers AJ (2009) The potential role and application of PARP inhibitors in cancer treatment. Br Med Bull 89: 23–40 - PubMed
-
- Dantzer F, Schreiber V, Niedergang C, Trucco C, Flatter E, De La Rubia G, Oliver J, Rolli V, Menissier-de Murcia J, de Murcia G (1999) Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie 81: 69–75 - PubMed
-
- Eigentler TK, Caroli UM, Radny P, Garbe C (2003) Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 4: 748–759 - PubMed
-
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361: 123–134 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

