The structural origin of second harmonic generation in fascia
- PMID: 21326632
- PMCID: PMC3028495
- DOI: 10.1364/BOE.2.000026
The structural origin of second harmonic generation in fascia
Abstract
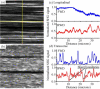
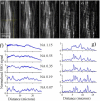
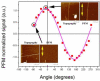
Fascia tissue is rich in collagen type I proteins and can be imaged by second harmonic generation (SHG) microscopy. While identifying the overall alignment of the collagen fibrils is evident from those images, the tridimensional structural origin for the observation of SHG signal is more complex than it apparently seems. Those images reveal that the noncentrosymmetric (piezoelectric) structures are distributed heterogeneously on spatial dimensions inferior to the resolution provided by the nonlinear optical microscope (sub-micron). Using piezoresponse force microscopy (PFM), we show that an individual collagen fibril has a noncentrosymmetric structural organization. Fibrils are found to be arranged in nano-domains where the anisotropic axis is preserved along the fibrillar axis, while across the collagen sheets, the phase of the second order nonlinear susceptibility is changing by 180 degrees between adjacent nano-domains. This complex architecture of noncentrosymmetric nano-domains governs the coherent addition of 2ω light within the focal volume and the observed features in the SHG images taken in fascia.
Keywords: (180.4315) Nonlinear microscopy; (190.4160) Multiharmonic generation.
Figures



References
LinkOut - more resources
Full Text Sources
