Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse
- PMID: 21326862
- PMCID: PMC3033905
- DOI: 10.1371/journal.pone.0016789
Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse
Abstract
Background: Human amniotic membrane-derived mesenchymal stem cells (hAMCs) have the potential to reduce heart and lung fibrosis, but whether could reduce liver fibrosis remains largely unknown.
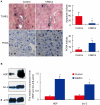
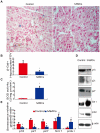
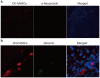
Methodology/principal findings: Hepatic cirrhosis model was established by infusion of CCl₄ (1 ml/kg body weight twice a week for 8 weeks) in immunocompetent C57Bl/6J mice. hAMCs, isolated from term delivered placenta, were infused into the spleen at 4 weeks after mice were challenged with CCl₄. Control mice received only saline infusion. Animals were sacrificed at 4 weeks post-transplantation. Blood analysis was performed to evaluate alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Histological analysis of the livers for fibrosis, hepatic stellate cells activation, hepatocyte apoptosis, proliferation and senescence were performed. The donor cell engraftment was assessed using immunofluorescence and polymerase chain reaction. The areas of hepatic fibrosis were reduced (6.2%±2.1 vs. control 9.6%±1.7, p<0.05) and liver function parameters (ALT 539.6±545.1 U/dl, AST 589.7±342.8 U/dl,vs. control ALT 139.1±138.3 U/dl, p<0.05 and AST 212.3±110.7 U/dl, p<0.01) were markedly ameliorated in the hAMCs group compared to control group. The transplantation of hAMCs into liver-fibrotic mice suppressed activation of hepatic stellate cells, decreased hepatocyte apoptosis and promoted liver regeneration. More interesting, hepatocyte senescence was depressed significantly in hAMCs group compared to control group. Immunofluorescence and polymerase chain reaction revealed that hAMCs engraftment into host livers and expressed the hepatocyte-specific markers, human albumin and α-fetoproteinran.
Conclusions/significance: The transplantation of hAMCs significantly decreased the fibrosis formation and progression of CCl₄-induced cirrhosis, providing a new approach for the treatment of fibrotic liver disease.
Conflict of interest statement
Figures



References
-
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607. - PubMed
-
- Kisseleva T, Gigante E, Brenner DA. Recent advances in liver stem cell therapy. Curr Opin Gastroenterol. 2010;26:395–402. - PubMed
-
- Huang YW, Hu JT, Yang SS. Hepatology; 2010. Complications of alcoholic liver cirrhosis: Active assessment by endoscopy and sonography. - PubMed
-
- Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, et al. American Association for the Study of Liver Diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology. 2010;52:349–359. - PubMed
-
- Park JK, Ki MR, Lee HR, Hong IH, Ji AR, et al. Vitamin C deficiency attenuates liver fibrosis by way of up-regulated peroxisome proliferator-activated receptor-gamma expression in senescence marker protein 30 knockout mice. Hepatology. 2010;51:1766–1777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

