Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates
- PMID: 21326877
- PMCID: PMC3034725
- DOI: 10.1371/journal.pone.0016622
Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates
Abstract
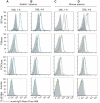
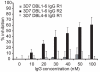
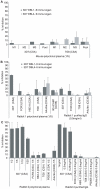
The high molecular weight, multidomain VAR2CSA protein mediating adhesion of Plasmodium falciparum-infected erythrocytes in the placenta is the leading candidate for a pregnancy malaria vaccine. However, it has been difficult so far to generate strong and consistent adhesion blocking antibody responses against most single-domain VAR2CSA immunogens. Recent advances in expression of the full-length recombinant protein showed it binds with much greater specificity and affinity to chondroitin sulphate A (CSA) than individual VAR2CSA domains. This raises the possibility that a specific CSA binding pocket(s) is formed in the full length antigen and could be an important target for vaccine development. In this study, we compared the immunogenicity of a full-length VAR2CSA recombinant protein containing all six Duffy binding-like (DBL) domains to that of a three-domain construct (DBL4-6) in mice and rabbits. Animals immunized with either immunogen acquired antibodies reacting with several VAR2CSA individual domains by ELISA, but antibody responses against the highly conserved DBL4 domain were weaker in animals immunized with full-length DBL1-6 recombinant protein compared to DBL4-6 recombinant protein. Both immunogens induced cross-reactive antibodies to several heterologous CSA-binding parasite lines expressing different VAR2CSA orthologues. However, antibodies that inhibited adhesion of parasites to CSA were only elicited in rabbits immunized with full-length immunogen and inhibition was restricted to the homologous CSA-binding parasite. These findings demonstrate that partial and full-length VAR2CSA immunogens induce cross-reactive antibodies, but inhibitory antibody responses to full-length immunogen were highly allele-specific and variable between animal species.
Conflict of interest statement
Figures
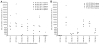
References
-
- Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, et al. The sick placenta-the role of malaria. Placenta. 2004;25:359–78. - PubMed
-
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. - PubMed
-
- Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

