The cellular response to DNA damage: a focus on MDC1 and its interacting proteins
- PMID: 21326949
- PMCID: PMC3030693
- DOI: 10.4161/nucl.1.2.11176
The cellular response to DNA damage: a focus on MDC1 and its interacting proteins
Abstract
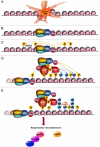
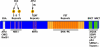
The DNA damage response (DDR) is comprised of a network of proteins that respond to DNA damage. Mediator of DNA Damage Checkpoint 1 (MDC1) plays an early and important role in the DDR. Recent data show that MDC1 binds multiple proteins that participate in various aspects of the DDR, positioning it at the core of the DDR. Furthermore, interactions with non-DDR proteins were also revealed, suggesting novel roles for MDC1.In this review we provide a comprehensive overview of all known MDC1-binding proteins and discuss their role. We present these binding partners according to their function, thereby providing the reader with a detailed and updated overview of the cellular response to DNA damage. We discuss more recent findings in detail and conclude by presenting the challenges the field faces in the future.
Keywords: DNA damage response; DNA repair; MDC1; double-strand breaks; genomic stability; phosphorylation; ubiquitination.
Figures
References
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. - PubMed
-
- Harper JW, Elledge SJ. The DNA damage response: Ten years after. Molecular Cell. 2007;28:739–745. - PubMed
-
- Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. - PubMed
-
- Petrini J, Stracker T. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 2003;13:458–462. - PubMed
-
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
