Mucin-bacterial interactions in the human oral cavity and digestive tract
- PMID: 21327032
- PMCID: PMC3023607
- DOI: 10.4161/gmic.1.4.12778
Mucin-bacterial interactions in the human oral cavity and digestive tract
Abstract
Mucins are a family of heavily glycosylated proteins that are the major organic components of the mucus layer, the protective layer covering the epithelial cells in many human and animal organs, including the entire gastro-intestinal tract. Microbes that can associate with mucins benefit from this interaction since they can get available nutrients, experience physico-chemical protection and adhere, resulting in increased residence time. Mucin-degrading microorganisms, which often are found in consortia, have not been extensively characterized as mucins are high molecular weight glycoproteins that are hard to study because of their size, complexity and heterogeneity. The purpose of this review is to discuss how advances in mucus and mucin research, and insight in the microbial ecology promoted our understanding of mucin degradation. Recent insight is presented in mucin structure and organization, the microorganisms known to use mucin as growth substrate, with a specific attention on Akkermansia muciniphila, and the molecular basis of microbial mucin degradation owing to availability of genome sequences.
Figures

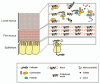

References
-
- Allen A. The structure and function of gastrointestinal mucus. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Vol. 1. New York Raven Press; 1981. pp. 617–639.
-
- Moore E. Physiology of intestinal and electrolyte absorption. American Gastroenterological Society. Baltimore: Milner-Fenwick; 1976.
-
- Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:1–19. - PubMed
-
- Allen A. Mucus—a protective secretion of complexity. Trends Biochem Sci. 1983;8:169–173.
LinkOut - more resources
Full Text Sources
Other Literature Sources
