Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver
- PMID: 21327394
- PMCID: PMC3052504
- DOI: 10.1007/s00418-011-0787-1
Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver
Abstract
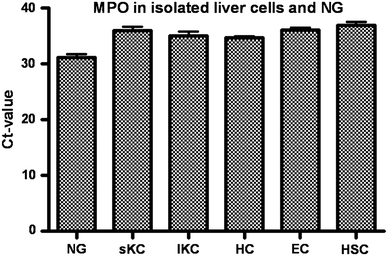
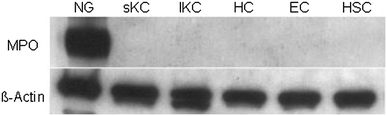
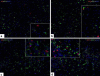
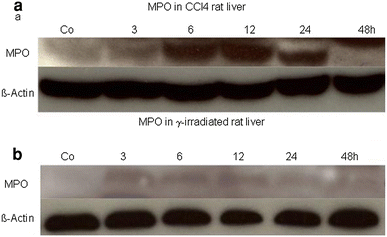
Myeloperoxidase (MPO) is involved in acute and chronic inflammatory diseases. The source of MPO in acute liver diseases is still a matter of debate. Therefore, we analysed MPO-gene expression on sections from normal and acutely damaged [carbon tetrachloride-(CCl(4)) or whole liver γ-Irradiation] rat liver by immunohistochemistry, real time PCR and Western blot analysis of total RNA and protein. Also total RNA and protein from isolated Kupffer cells, hepatic stellate cells, Hepatocytes, endothelial cells and neutrophil granulocytes (NG) was analysed by real time PCR and Western blot, respectively. Sections of acutely injured human liver were prepared for MPO and CD68 immunofluorescence double staining. In normal rat liver MPO was detected immunohistochemically and by immunofluorescence double staining only in single NG. No MPO was detected in isolated parenchymal and non-parenchymal cell populations of the normal rat liver. In acutely damaged rat liver mRNA of MPO increased 2.8-fold at 24 h after administration of CCl(4) and 3.3-fold at 3 h after γ-Irradiation and MPO was detected by immunofluorescence double staining only in elastase (NE) positive NGs but not in macrophages (ED1 or CD68 positive cells). Our results demonstrate that, increased expression of MPO in damaged rat and human liver is due to recruited elastase positive NGs.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

