Improving the follow-up of positive hemoccult screening tests: an electronic intervention
- PMID: 21327529
- PMCID: PMC3138585
- DOI: 10.1007/s11606-011-1639-3
Improving the follow-up of positive hemoccult screening tests: an electronic intervention
Abstract
Background: Four population-based studies of screening for CRC with fecal occult blood testing (FOBT) have shown that mortality can be significantly reduced. However, nearly half of all positive screening tests are not appropriately evaluated.
Objectives: We evaluated whether an electronic record intervention improved the follow-up of patients with a positive FOBT (FOBT+) result.
Design: We conducted a cluster randomized trial involving four Veteran's Affairs (VA) medical centers pair-matched by colonoscopy volume and randomized within the pair to receive the electronic intervention or usual care.
Participants: All patients with FOBT+ results at participating facilities during a matched pre- and post-intervention time period.
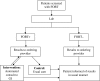
Interventions: In the two intervention sites, an electronic consult that imported relevant clinical information was automatically submitted to the gastroenterology (GI) clinic for all FOBT+ patients at the time the result was recorded in the laboratory. In both intervention and control sites (usual care), PCPs continued to be notified of FOBT+ results in the usual manner
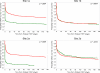
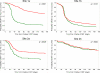
Measures: Pre- and post-intervention changes in the proportion of FOBT+ patients having: (1) a GI consult or (2) a GI consult plus complete diagnostic evaluation (CDE) of the colon within 30, 90 and 180 days were compared across intervention and control sites. Log rank tests were used to determine statistical significance.
Results: The 30-, 90- and 180-day GI consult rates improved 21-33 % (p < 0.001) among intervention sites, but did not change in the usual care sites. Thirty-, 90- and 180-day CDE rates improved 9-31% (p < 0.03) in intervention sites, but did not significantly change in the usual care sites. Time to GI consult and CDE decreased significantly over time in the intervention sites (p < 0.001), but remained unchanged in the usual care sites.
Conclusions: The relatively simple electronic intervention evaluated can significantly improve the follow-up of FOBT+ results. Interventions such as this could improve patient care and may be applicable to other practice settings, as well as other types of tests.
Figures


Comment in
-
Electronic medical records and improving the quality of the screening process.J Gen Intern Med. 2011 Jul;26(7):683-4. doi: 10.1007/s11606-011-1722-9. J Gen Intern Med. 2011. PMID: 21538167 Free PMC article. No abstract available.
References
-
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

