Treatment for Lambert-Eaton myasthenic syndrome
- PMID: 21328260
- PMCID: PMC7003613
- DOI: 10.1002/14651858.CD003279.pub3
Treatment for Lambert-Eaton myasthenic syndrome
Abstract
Background: Lambert-Eaton myasthenic syndrome (LEMS) is an autoimmune disorder of neuromuscular transmission. Treatments attempt to overcome the harmful autoimmune process, or improve residual neuromuscular transmission
Objectives: The objective was to examine the efficacy of treatment in Lambert-Eaton myasthenic syndrome.
Search strategy: We searched the Cochrane Neuromuscular Disease Group Specialized Register (12 October 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (12 October 2010, Issue 4 2010 in the Cochrane Library), MEDLINE (January 1966 to September 2010) and EMBASE (January 1980 to September 2010).
Selection criteria: All randomised or quasi-randomised trials of adults and children with a diagnosis of Lambert-Eaton myasthenic syndrome, with or without small-cell lung cancer, receiving any form of pharmacological or physical treatment.
Data collection and analysis: All authors independently assessed studies for inclusion and extracted data. Study authors were contacted for missing information when possible.
Main results: Four controlled trials of 3,4-diaminopyridine compared with placebo in a total of 54 participants with Lambert-Eaton myasthenic syndrome were eligible: three cross-over trials and one parallel group. Two were added at this update. One of these trials also assessed pyridostigmine in conjunction with 3,4-diaminopyridine. A further cross-over trial compared intravenous immunoglobulin (IVIg) to placebo in nine participants.Four trials of 3,4-diaminopyridine reported significant improvement in the primary outcome, muscle strength score, or myometric limb measurement for between hours and a week following treatment, and significant improvement in resting compound muscle action potential (CMAP) amplitude following 3,4-diaminopyridine, compared with placebo.A meta-analysis of the primary endpoint showed Quantitative Myasthenia Gravis (QMG) muscle score assessed between three and eight days was likely to improve by a mean of 2.44 points (95% confidence interval 3.6 to 1.22). Meta-analysis of the secondary endpoint CMAP amplitude also showed a mean improvement of 1.36 mV (95% confidence interval 0.99 to 1.72) over the same period. The risk of bias was determined to be low, and quality of evidence moderate to high.A single cross-over trial reported significant improvement in myometric limb strength and non-significant improvement in mean resting CMAP amplitude with IVIg compared to placebo. Clinical improvement lasted for up to eight weeks.
Authors' conclusions: Limited but moderate to high quality evidence from randomised controlled trials showed that over days 3,4-diaminopyridine, or for up to 8 weeks IVIg, improved muscle strength scores and CMAP amplitudes in participants with Lambert-Eaton myasthenic syndrome. There are insufficient data at present to quantify this effect. Other possible treatments have not been tested in randomised controlled trials.
Conflict of interest statement
Michael Keogh ‐ none known.
Saam Sedehizadeh ‐ none known.
Paul Maddison has received an honorarium from BioMarin, manufacturers of Firdapse, for an advisory meeting..
Figures
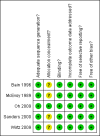
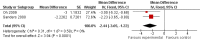




Update of
-
Treatment for Lambert-Eaton myasthenic syndrome.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003279. doi: 10.1002/14651858.CD003279.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2011 Feb 16;(2):CD003279. doi: 10.1002/14651858.CD003279.pub3. PMID: 15846654 Updated.
Similar articles
-
Treatment for Lambert-Eaton myasthenic syndrome.Cochrane Database Syst Rev. 2003;(2):CD003279. doi: 10.1002/14651858.CD003279. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003279. doi: 10.1002/14651858.CD003279.pub2. PMID: 12804456 Updated.
-
Treatment for Lambert-Eaton myasthenic syndrome.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003279. doi: 10.1002/14651858.CD003279.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2011 Feb 16;(2):CD003279. doi: 10.1002/14651858.CD003279.pub3. PMID: 15846654 Updated.
-
Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy.Cochrane Database Syst Rev. 2017 May 8;5(5):CD003280. doi: 10.1002/14651858.CD003280.pub5. Cochrane Database Syst Rev. 2017. PMID: 28481421 Free PMC article.
-
Interventions for eye movement disorders due to acquired brain injury.Cochrane Database Syst Rev. 2018 Mar 5;3(3):CD011290. doi: 10.1002/14651858.CD011290.pub2. Cochrane Database Syst Rev. 2018. PMID: 29505103 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Autoimmune Channelopathies at Neuromuscular Junction.Front Neurol. 2019 May 17;10:516. doi: 10.3389/fneur.2019.00516. eCollection 2019. Front Neurol. 2019. PMID: 31156543 Free PMC article. Review.
-
Guideline for the management of myasthenic syndromes.Ther Adv Neurol Disord. 2023 Dec 26;16:17562864231213240. doi: 10.1177/17562864231213240. eCollection 2023. Ther Adv Neurol Disord. 2023. PMID: 38152089 Free PMC article. Review.
-
Emergency Neurological Life Support: Acute Non-traumatic Weakness.Neurocrit Care. 2015 Dec;23 Suppl 2:S23-47. doi: 10.1007/s12028-015-0160-7. Neurocrit Care. 2015. PMID: 26438455 Review.
-
Management of Paraneoplastic Syndromes in the Era of Immune Checkpoint Inhibitors.Curr Treat Options Oncol. 2024 Jan;25(1):42-65. doi: 10.1007/s11864-023-01157-1. Epub 2024 Jan 10. Curr Treat Options Oncol. 2024. PMID: 38198120 Review.
-
Therapeutic Plasma Exchange: For Cancer Patients.Cancer Manag Res. 2022 Feb 2;14:411-425. doi: 10.2147/CMAR.S340472. eCollection 2022. Cancer Manag Res. 2022. PMID: 35140519 Free PMC article. Review.
References
References to studies included in this review
Bain 1996 {published data only}
-
- Bain PG, Motomura M, Newsom‐Davis J, Misbah SA, Chapel HM, Lee ML, et al. Effects of intravenous immunoglobulin on muscle weakness and calcium‐channel autoantibodies in the Lambert‐Eaton myasthenic syndrome. Neurology 1996;47(3):678‐83. [PUBMED: 8797464 ] - PubMed
McEvoy 1989 {published data only}
-
- McEvoy KE, Windebank AJ, Daube JR. 3,4‐diaminopyridine in Lambert‐Eaton myasthenic syndrome. Annals of Neurology 1988;24(1):122.
-
- McEvoy KM, Windebank AJ, Daube JR, Low PA. 3,4‐Diaminopyridine in the treatment of Lambert‐Eaton myasthenic syndrome. New England Journal of Medicine 1989;321(23):1567‐71. [PUBMED: 2555713] - PubMed
Oh 2009 {published and unpublished data}
-
- Oh SJ, Claussen GG, Hatanaka Y, Morgan MB. 3,4 ‐ Diaminopyridine is more effective than placebo in a randomized, double‐blind, cross‐over drug study in LEMS. Muscle and Nerve 2009;40(5):795‐800. [PUBMED: 19722254 ] - PubMed
Sanders 2000 {published data only}
-
- Sanders DB, Massey JM, Sanders LL, Edwards LJ. A randomized trial of 3,4‐diaminopyridine in Lambert‐Eaton myasthenic syndrome. Neurology 2000;54(3):603‐7. [PUBMED: 10680790] - PubMed
Wirtz 2009 {published and unpublished data}
-
- Wirtz PW, Verschuuren JJ, Dijk JG, Kam ML, Schoemaker RC, Hasselt JGC, Titulaer MJ, Tjaden UR, Hartigh J, Gerven JM. Efficacy of 3,4‐diaminopyridine and pyridostigmine in the treatment of Lambert‐Eaton myasthenic syndrome: a randomized, double‐blind, placebo‐controlled, crossover study.. Clinical Pharmacology and Therpautics July 2009;86(1):44‐8. [PUBMED: 19357643] - PubMed
Additional references
AAEM 2001a
-
- AAEM Quality Assurance Committee. American Association of Electrodiagnostic Medicine. Practice parameter for repetitive nerve stimulation and single fiber EMG evaluation of adults with suspected myasthenia gravis or Lambert‐Eaton myasthenic syndrome: summary statement. Muscle and Nerve 2001;24(9):1236‐8. [PUBMED: 11494280] - PubMed
AAEM 2001b
-
- AAEM Quality Assurance Committee. American Association of Electrodiagnostic Medicine. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert‐Eaton myasthenic syndrome. Muscle and Nerve 2001;24(9):1239‐47. [PUBMED: 11494281] - PubMed
Agoston 1978
-
- Agoston S, Weerden T, Westra P, Broekert A. Effects of 4‐aminopyridine in Eaton Lambert Syndrome. British Journal of Anaesthesia 1978;50(4):383‐5. [PUBMED: 656256] - PubMed
Barohn 1998
-
- Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis score. Annals of the New York Academy of Sciences 1998;841:769‐72. [PUBMED: 9668327] - PubMed
Besinger 1983
-
- Besinger UA, Toyka KV, Hömberg M, Heininger K, Hohlfeld R, Fateh‐Moghadam A. Myasthenia gravis: long‐term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology 1983;33(10):1316‐21. [PUBMED: 6684226] - PubMed
BioMarin 2010
-
- BioMarin Europe Ltd. Summary of Product Characteristics. Firdapse® (amifampridine). Date of revision of the text February 2010.
Bird 1992
-
- Bird SJ. Clinical and electrophysiologic improvement in Lambert‐Eaton syndrome with intravenous immunoglobulin therapy. Neurology 1992;42(7):1422‐3. [PUBMED: 1620360] - PubMed
Blumhardt 1977
Dalakas 1999
-
- Dalakas MC. Intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: present status and practical therapeutic guidelines. Muscle and Nerve 1999;22(11):1479‐97. [PUBMED: 10514226] - PubMed
Fukunaga 1983
-
- Fukunaga H, Engel AG, Lang B, Newsom‐Davis J, Vincent A. Passive transfer of Lambert‐Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proceedings of the National Academy of Sciences of the United States of America 1983;80(24):7636‐40. [PUBMED: 6584877] - PMC - PubMed
Higgins 2008
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008.
Lambert 1956
-
- Lambert EH, Eaton LM, Rooke ED. Defect of neuromuscular conduction associated with malignant neoplasms. American Journal of Physiology 1956;187(3):612‐3.
Lambert 1966
-
- Lambert EH. Defects of neuromuscular transmission in syndromes other than myasthenia gravis. Annals of the New York Academy of Sciences 1966;135(1):367‐84. [PUBMED: 5221351] - PubMed
Lambert 1971
-
- Lambert EH, Elmqvist D. Quantal components of end‐plate potentials in the myasthenic syndrome. Annals of the New York Academy of Sciences 1971;183:183‐99. [PUBMED: 4330759] - PubMed
Lang 1981
-
- Lang B, Newsom‐Davis J, Wray D, Vincent A, Murray N. Autoimmune aetiology for myasthenic (Eaton‐Lambert) syndrome. Lancet 1981;2(8240):224‐6. [PUBMED: 6114283] - PubMed
Lang 1983
Lang 1987
Lechat 1968
-
- Lechat P, Deysson G, Lemeignan M, Adolphe M. Comparison of acute toxicity of some aminopyridines in vivo (mice) and in vitro (tissue culture) [Toxicité aigue compareé de quelques aminopyridines in vivo (souris) et in vitro (cultures cellulaires)]. Annales Pharmaceutiques Francaises 1968;26(5):345‐9. [PUBMED: 5716404] - PubMed
Lemeignan 1971
-
- Lemeignan M. Pharmacological approach to the study of convulsive action mechanism of amino‐4 pyridine [Abord pharmacologique de l'étude du mécanisme de l'action convulsivante de l'amino‐4 pyridine]. Therapie 1971;26(5):927‐40. [PUBMED: 4400759] - PubMed
Lemeignan 1982
-
- Lemeignan M, Millart H, Letteron N, Liamable D, Josso J, Choisy H, et al. The ability of 4‐aminopyridine and 3,4‐diaminopyridine to cross the blood‐brain barrier can account for their difference in toxicity. In: Lechat P, Thesleff S, Bowman WC editor(s). Aminopyridines and similarly acting drugs: Effects on nerves, muscles and synapses. Advances in the Biosciences. Vol. 35, Oxford: Pergamon Press, 1982:222‐9.
Lennon 1995
-
- Lennon VA, Kryzer TJ, Griesmann GE, O'Suilleabhain PE, Windebank AJ, Woppmann A, et al. Calcium‐channel antibodies in the Lambert‐Eaton syndrome and other paraneoplastic syndromes. New England Journal of Medicine 1995;332(22):1467‐74. [PUBMED: 7739683] - PubMed
Lundh 1977
Lundh 1978
-
- Lundh H. Effects of 4‐aminopyridine on neuromuscular transmission. Brain Research 1978;153(2):307‐18. [PUBMED: 210882] - PubMed
Lundh 1983
Lundh 1993
-
- Lundh H, Nilsson O, Rosén I, Johansson S. Practical aspects of 3,4‐diaminopyridine treatment of the Lambert‐Eaton myasthenic syndrome. Acta Neurologica Scandinavica 1993;88(2):136‐40. [PUBMED: 8213058] - PubMed
Molgo 1980
-
- Molgó J, Lundh H, Thesleff S. Potency of 3,4‐diaminopyridine and 4‐aminopyridine on mammalian neuromuscular transmission and the effect of pH changes. European Journal of Pharmacology 1980;61(1):25‐34. [PUBMED: 6101553] - PubMed
Motomura 1995
Muchnik 1997
-
- Muchnik S, Losavio AS, Vidal A, Cura L, Mazia C. Long‐term follow‐up of Lambert‐Eaton syndrome treated with intravenous immunoglobulin. Muscle and Nerve 1997;20(6):674‐8. [PUBMED: 9149073] - PubMed
Murray 1981
-
- Murray NM, Newsom‐Davis J. Treatment with oral 4‐aminopyridine in disorders of neuromuscular transmission. Neurology 1981;31(3):265‐71. [PUBMED: 6259555] - PubMed
Newsom‐Davis 1984
-
- Newsom‐Davis J, Murray NM. Plasma exchange and immunosuppressive drug treatment in the Lambert‐Eaton myasthenic syndrome. Neurology 1984;34(4):480‐5. [PUBMED: 6322050] - PubMed
O'Neill 1988
-
- O'Neill JH, Murray NM, Newsom‐Davis J. The Lambert‐Eaton myasthenic syndrome: a review of 50 cases. Brain 1988;111(Pt 3):577‐96. [PUBMED: 2838124] - PubMed
Oh 1973
-
- Oh SJ, Kim KW. Guanidine hydrochloride in the Eaton‐Lambert syndrome. Electrophysiologic improvement. Neurology 1973;23(10):1084‐90. [PUBMED: 4355063] - PubMed
Oh 1997
-
- Oh SJ, Kim DS, Head TC, Claussen GC. Low‐dose guanidine and pyridostigmine: relatively safe and effective long‐term symptomatic therapy in Lambert‐Eaton myasthenic syndrome. Muscle and Nerve 1997;20(9):1146‐52. [PUBMED: 9270671] - PubMed
Roberts 1985
-
- Roberts A, Perera S, Lang B, Vincent A, Newsom‐Davis J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature 1985;317(6039):737‐9. [PUBMED: 2414666] - PubMed
Takano 1994
-
- Takano H, Tanaka M, Koike R, Nagai H, Arakawa M, Tsuji S. Effect of intravenous immunoglobulin in Lambert‐Eaton myasthenic syndrome with small‐cell cancer: correlation with the titer of anti‐voltage‐gated calcium channel antibody. Muscle and Nerve 1994;17(9):1073‐5. [PUBMED: 8065398] - PubMed
Tindall 1987
-
- Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G. Preliminary results of a double‐blind, randomized, placebo‐controlled trial of cyclosporine in myasthenia gravis. New England Journal of Medicine 1987;316(12):719‐24. [PUBMED: 3547126] - PubMed
UKMi Pharmacists 2010
-
- UK Medicines information (UKMi) Pharmacists for NHE professionals. What is the difference between amifampridine and 3,4‐diaminopyridine base for Lambert‐Eaton myasthenic syndrome in adults?. Accessed via www.nelm.nhs.uk May 24th 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

