Thymic peptides for treatment of cancer patients
- PMID: 21328265
- PMCID: PMC6481824
- DOI: 10.1002/14651858.CD003993.pub3
Thymic peptides for treatment of cancer patients
Abstract
Background: Purified thymus extracts (pTE) and synthetic thymic peptides (sTP) are thought to enhance the immune system of cancer patients in order to fight the growth of tumour cells and to resist infections due to immunosuppression induced by the disease and antineoplastic therapy.
Objectives: To evaluate the effectiveness of pTE and sTP for the management of cancer.
Search strategy: We searched CENTRAL (The Cochrane Library 2010, Issue 3), MEDLINE, EMBASE, AMED, BIOETHICSLINE, BIOSIS, CATLINE, CISCOM, HEALTHSTAR, HTA, SOMED and LILACS (to February 2010).
Selection criteria: Randomised trials of pTE or sTP in addition to chemotherapy or radiotherapy, or both, compared to the same regimen with placebo or no additional treatment in adult cancer patients.
Data collection and analysis: Two authors independently extracted data from published trials. We derived odds ratios (OR) from overall survival (OS) and disease-free survival (DFS) rates, tumour response (TR) rates, and rates of adverse effects (AE) related to antineoplastic treatments. We used a random-effects model for meta-analysis.
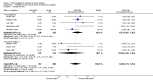
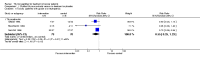
Main results: We identified 26 trials (2736 patients). Twenty trials investigated pTE (thymostimulin or thymosin fraction 5) and six trials investigated sTP (thymopentin or thymosin α(1)). Twenty-one trials reported results for OS, six for DFS, 14 for TR, nine for AE and 10 for safety of pTE and sTP. Addition of pTE conferred no benefit on OS (RR 1.00, 95% CI 0.79 to 1.25); DFS (RR 0.97, 95% CI 0.82 to 1.16); or TR (RR 1.07, 95% CI 0.92 to 1.25). Heterogeneity was moderate to high for all these outcomes. For thymosin α(1) the pooled RR for OS was 1.21 (95% CI 0.94 to 1.56, P = 0.14), with low heterogeneity; and 3.37 (95% CI 0.66 to 17.30, P = 0.15) for DFS, with moderate heterogeneity. The pTE reduced the risk of severe infectious complications (RR 0.54, 95% CI 0.38 to 0.78, P = 0.0008; I² = 0%). The RR for severe neutropenia in patients treated with thymostimulin was 0.55 (95% CI 0.25 to 1.23, P = 0.15). Tolerability of pTE and sTP was good. Most of the trials had at least a moderate risk of bias.
Authors' conclusions: Overall, we found neither evidence that the addition of pTE to antineoplastic treatment reduced the risk of death or disease progression nor that it improved the rate of tumour responses to antineoplastic treatment. For thymosin α(1), there was a trend for a reduced risk of dying and of improved DFS. There was preliminary evidence that pTE lowered the risk of severe infectious complications in patients undergoing chemotherapy or radiotherapy.
Conflict of interest statement
None known. It is certified that there are no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (for example employment, consultancy, stock ownership, honoraria).
Figures






Update of
- doi: 10.1002/14651858.CD003993.pub2
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2022 Aug 22;8(8):CD010260. doi: 10.1002/14651858.CD010260.pub3. Cochrane Database Syst Rev. 2022. PMID: 35994243 Free PMC article.
-
Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer.Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD007047. doi: 10.1002/14651858.CD007047.pub2. Cochrane Database Syst Rev. 2017. PMID: 28654140 Free PMC article.
Cited by
-
Proteomic profiling of the endogenous peptides of MRSA and MSSA.PeerJ. 2021 Nov 24;9:e12508. doi: 10.7717/peerj.12508. eCollection 2021. PeerJ. 2021. PMID: 34900427 Free PMC article.
-
Novel strategy of sirolimus plus thymalfasin and huaier granule on tumor recurrence of hepatocellular carcinoma beyond the UCSF criteria following liver transplantation: A single center experience.Oncol Lett. 2018 Oct;16(4):4407-4417. doi: 10.3892/ol.2018.9226. Epub 2018 Jul 27. Oncol Lett. 2018. PMID: 30214575 Free PMC article.
-
Thymopentin-Mediated Inhibition of Cancer Stem Cell Stemness Enhances the Cytotoxic Effect of Oxaliplatin on Colon Cancer Cells.Front Pharmacol. 2022 Feb 15;13:779715. doi: 10.3389/fphar.2022.779715. eCollection 2022. Front Pharmacol. 2022. PMID: 35242031 Free PMC article.
-
Prevention of hepatocellular carcinoma: potential targets, experimental models, and clinical challenges.Curr Cancer Drug Targets. 2012 Nov 1;12(9):1129-59. Curr Cancer Drug Targets. 2012. PMID: 22873223 Free PMC article. Review.
-
Safety signals of albumin-bound paclitaxel: Data mining of the Food and Drug Administration adverse event reporting system.Indian J Pharmacol. 2023 May-Jun;55(3):167-173. doi: 10.4103/ijp.ijp_640_22. Indian J Pharmacol. 2023. PMID: 37555411 Free PMC article.
References
References to studies included in this review
Airoldi 1987 {published data only}
-
- Airoldi M, Gabriele P, Penani F, Brando V, Melano A. Chemioterapia versus chemioimmunoterapia con Timostimulina nei carcinomi avanzati del cavo orale: risultati clinici di uno studio randomizzato. Recenti Progressi in Medicina 1987;78 Suppl 6:67‐70.
Bedikian 1984 {published data only}
-
- Bedikian AY, Patt YZ, Murphy WK, Umsawasadi T, Carr DT, Hersh EM, et al. Prospective evaluation of thymosin fraction V immunotherapy in patients with non‐small cell lung cancer receiving vindesine, doxorubicin, and cisplatin (VAP) chemotherapy. American Journal of Clinical Oncology 1984;7(5):399‐404. - PubMed
Canovas 1988 {published data only}
-
- Canovas Fernandez A, Gonzalez de Zarate P, Alonso Alonso J, Aguirre Errasti C. [Thymostimulin as an adjuvant to antineoplastic chemotherapy. Experience with patients with myeloma and lymphoma]. Revista Clinica Espanola 1988;183(7):340‐3. - PubMed
Canovas 1991 {published data only}
-
- Canovas Fernandez A, Alonso Alonso J, Gonzalez de Zarate P, Garcia Masdevall MD, Rinon Martinez‐Gallo M, Aguirre Errasti C. [The use of thymostimulin in lymphoma and myeloma patients]. Anales de Medicina Interna 1991;8(2):69‐73. - PubMed
Cheng 2004 {published data only}
-
- Cheng S, Mengchao W, Han C, Feng S, Jiahe Y, Wenming C, et al. Combination transcatheter hepatic arterial chemoembolization with thymosin alpha1 on recurrence prevention of hepatocellular carcinoma. Hepatogastroenterology 2004;51(59):1445‐7. - PubMed
Cohen 1979 {published data only}
-
- Chretien PB, Lipson SD, Makuch R, Kenady DE, Cohen MH, Minna JD. Thymosin in cancer patients: in vitro effects and correlations with clinical response to thymosin immunotherapy. Cancer Treatment Reports 1978;62(11):1787‐90. - PubMed
-
- Cohen MH, Chretien PB, Ihde DC, Fossieck BE, Makuch R, Bunn PA, et al. Thymosin fraction V and intensive combination chemotherapy. Journal of the American Medical Association 1979;241:1813‐5. - PubMed
-
- Lipson SD, Chretien PB, Makuch R, Kenady DE, Cohen MH. Thymosin immunotherapy in patients with small cell carcinoma of the lung: correlation of in vitro studies with clinical course. Cancer 1979;43(3):863‐70. - PubMed
Del Giacco 1988 {published data only}
-
- Giacco GS, Mantovani G, Cengiarotti L, Puxeddu G, Pischedda A, Tucci A, et al. Secondary immunodeficiency in advanced lung cancer: effects of chemotherapy plus thymostimulin in immunodepressed patients: updating results. International Journal of Tissue Reactions 1984;6(6):499‐504. - PubMed
-
- Giacco GS, Mantovani G, Pilidi G, et al. Thymic factors in lung cancer. In: Nagel G, Schioppacassi G, Schuff‐Werner P editor(s). Thymic hormones in oncology. Roma: Ares Serono Symposia, 1988:149‐56.
De Serdio 1997 {published data only}
-
- Serdio JL, Villar A, Alvarez IE, Gil‐Cubelo JA, Suner M, Hernandez R, et al. [The effects of thymostimulin in a protocol of concurrent hyperfractionated carboplatin and irradiation]. Acta Otorrinolaringologica Espanola 1997;48(6):487‐92. - PubMed
Federico 1995 {published data only}
-
- Federico M, Gobbi PG, Mretti G, Avanzini P, Renzo N, Cavanna L, et al. Effects of thymostimulin with combination chemotherapy in patients with aggressive Non‐Hodgkin lymphoma. A report from the Italian Lymphoma Study Group (GISL). American Journal of Clinical Oncology 1995;18(1):8‐14. - PubMed
Gebbia 1994 {published data only}
-
- Gebbia V, Valenza R, Testa A, Cannata G, Borsellino N, Gebbia N. A prospective randomized trial of thymopentin versus granulocyte‐colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Research 1994;14(2B):731‐4. - PubMed
Gish 2009 {published data only}
GISOT 1987 {published data only}
-
- GISOT (Gruppo Italiano di Studio Sugli Ormoni Timici). [Efficacy and tolerability of thymopoietin in patients at risk of infection]. Recenti Progressi in Medicina 1987;78(7‐8):358‐64. - PubMed
Gonnelli 1995 {published data only}
-
- Gonnelli S, Petrioli R, Cepollaro C, Palmieri R, Aquino A, Gennari C. Thymostimulin in association with chemotherapy in breast cancer patients with bone metastases. Clinical Drug Investigation 1995;9(2):79‐87.
Guzman 1988 {published data only}
-
- Guzman Machado CA, Verruno L, Ribas MC. Adjuvant therapy with thymic factors in solid tumours. In: Nagel G, Schioppacassi G, Schuff‐Werner P editor(s). Thymic Hormones in Oncology. Roma: Ares serono Symposia, 1988:121‐32.
Iaffaioli 1994 {published data only}
-
- Iaffaioli RV, Caponigro F, Facchini G, Tortoriello A, Panelli G, Muto P, et al. Immunological and clinical effects of thymostimulin in patients with locally advanced non‐small‐cell lung cancer treated with combined chemoradiotherapy. Drug Investigation 1994;7:209‐14.
Luzi 1984 {published data only}
-
- Luzi G, Tropea F, Siminara R, et al. Clinical and immunological evaluation in non‐resectable lung cancer patients treated with thymostimulin. Serono Symposia Publications 1984;16:309‐20.
Macchiarini 1989 {published data only}
-
- Macchiarini P, Danesi R, Tacca M, Angeletti CA. Effects of thymostimulin on chemotherapy‐induced toxicity and long‐term survival in small cell lung cancer patients. Anticancer Research 1989;9:193‐6. - PubMed
Maio 2010 {published and unpublished data}
-
- Camerini R, Mackiewicz A, Testori A, Trefzer U, Jassem J, Ferraresi V, et al. A large first‐line, randomized, dose‐finding, phase II study on thymosin‐alpha 1 (T‐alpha 1) plus dacarbazine (DTIC) with or without interferon‐alpha (IFN‐alpha) compared to DTIC plus IFN‐alpha in stage IV melanoma. Tumor response and survival results. Journal of Clinical Oncology 2007;25(18S):8535.
-
- Camerini R, Mackiewicz A, Testori A, Trefzer U, Jassem J, Ferraresi V, et al. A large first‐line, randomized, dose‐finding, phase II study on thymosin‐alpha 1 (T‐alpha 1) plus dacarbazine (DTIC) with or without interferon‐alpha (IFN‐alpha) compared to DTIC plus IFN‐alpha in stage IV melanoma. Tumor response and survival results. Powerpoint presentation ASCO Annual Meeting June 1‐5, 2007, Chicago.
-
- Maio M, Mackiewicz A, Testori A, Trefzer U, Ferraresi V, Jassem J, et al. Large randomized study of thymosin {alpha} 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. Journal of Clinical Oncology 2010;[Epub ahead of print]:1780‐7. - PubMed
Mantovani 1988 {published data only}
-
- Mantovani G, Coiana A, Zucca MV, Sanna GP, Floris C, Sulis E, et al. [Results of a controlled clinical protocol in the treatment of patients with operable breast carcinoma with positive axillary lymph nodes. Comparison of a chemotherapeutic regimen with a chemo‐immunotherapeutic regimen]. Recenti Progressi in Medicina 1988;79(12):513‐8. - PubMed
Mustacchi 1994 {published data only}
-
- Mustacchi G, Pavesi L, Milani S, Iaffaioli V, Caraco A, Comella G, et al. High‐dose folinic acid (FA) and fluorouracil (FU) plus or minus thymostimulin (TS) for treatment of metastatic colorectal cancer: results of a randomized multicenter clinical trial. Anticancer Research 1994;14:617‐20. - PubMed
Pavesi 1993 {published data only}
-
- Pavesi L. Fluorouracil (F), with and without high‐dose folinic acid (HDFA) plus epirubicin (E) and cyclophosphamide (C): FEC versus HDFA‐FEC plus or minus thymostimulin (TS) in metastatic breast cancer: results of a multicenter study. European Journal of Cancer 1993; Vol. 29 A Suppl:77.
Salvati 1984 {published data only}
-
- Salvati F, Pallotta G, Antilli A, Nunziati F, Marinis F, Lucchesi M. [MACC plus Thymostimulin (TP‐1 Serono) therapy of small cell bronchogenic carcinoma. Clinico‐immunologic evaluation of the results of a randomized trial]. Giornale Italiano di Chemiotherapia 1984;31:185‐9. - PubMed
Sanchiz 1996 {published data only}
-
- Sanchiz F, Milla A. A randomised study comparing granulocyte‐colony stimulating factor (G‐CSF) with G‐CSF plus thymostimulin in the treatment of heamatological toxicity in patients with advanced breast cancer after high dose mitoxantrone therapy. European Journal of Cancer 1996;32 A:52‐6. - PubMed
Scher 1988 {published data only}
-
- Scher HI, Shank B, Geller N, Pinsky C, Gralla R, Kelsen D, et al. Randomized trial of combined modality therapy with and without thymosin fraction V in the treatment of small cell lung cancer. Cancer Research 1988;48(6):1663‐70. - PubMed
Schulof 1985 {published data only}
-
- Schulof RS, Lloyd MJ, Cleary PA, Palaszynski SR, Mai DA, Cox JW, et al. A randomized trail to evaluate the immunorestorative properties of synthetic thymosin‐a in patients with lung cancer. Journal of Biological Response Modifiers 1985;4(2):147‐58. - PubMed
Wara 1981 {published data only}
-
- Wara WM, Neely MH, Amman AJ, Wara DW. Biologic modifications of immunologic parameters in head and neck cancer patients with thymosin fraction V. In: Goldstein AL, Chirigos MA editor(s). Lymphokines and Thymic Hormones: their potential utilization in cancer therapeutics. Raven Press, New York, 1981:257‐62.
References to studies excluded from this review
Azizi 1984 {published data only}
-
- Azizi E, Brenner HJ, Shoham J. Postoperative adjuvante behandlung von patienten mit malignem melanom durch die thymusfaktor thymostimulin. Arzneimittelforschung 1984;34(II):1043‐6.
Bernengo 1983 {published data only}
-
- Bernengo MG, Fra P, Lisa F, Meregalli M, Zina G. Thymostimulin therapy in melanoma patients: correlation of immunologic effects with clinical course. Clinical Immunology and Immunopathology 1983;28(3):311‐24. - PubMed
-
- Bernengo MG, Lisa F, Meregalli M, Matteis A, Zina G. The prognostic value of T‐lymphocyte levels in malignant melanoma. A five‐year follow‐up. Cancer 1983;52(10):1841‐8. - PubMed
Cartia 1990 {published data only}
-
- Cartia GL. [Evaluation of the effects of thymopentin on the incidence of leucopenia in patients treated with chemotherapy for breast carcinoma] Valutazione degli effetti di Timopentina sull`incidenza di leucopenia in pazienti trattate con chemioterapia per carcinom mammario. Minerva Medica 1990;81(11):815‐7. - PubMed
Chen 2000 {published data only}
-
- Chen J, Huang F, Zheng X, Chen L, Qin S, Li G, et al. Thymosin alpha 1 positively alters quality of life in chemotherapy treated patients. Proceedings American Society of Clinical Oncology 2000; Vol. 1.
De Maria 1993 {published data only}
-
- Maria D, Falchi AM, Armaroli L, Balli M, Bortolus R, Busutti L, et al. T‐lymphocyte subsets in cancer patients undergoing radiotherapy and their recovery after thymostimulin treatment. A cooperative study. RAYS 1993;18:438‐53. - PubMed
Denaro 1994 {published data only}
-
- Denaro A, Stivala F, Mazzarino MC. [Immunologic study on patients with head and neck cancer treated with thymopentin associated with surgery, chemotherapy and radiotherapy]. Acta Otorhinolaryngologica Italica 1994;14:611‐25. - PubMed
Holowiecki 1984 {published data only}
-
- Holowiecki J, Duraj M, Rudzka E, Jarczok K, Krawczyk M, Holowiecka B, et al. Cooperative randomized studies on the treatment of adult acute leukemia in Poland. A comparison of two remission induction regimes and two maintenance regimes for AML. Folia Haematologica 1984;111(2):201‐7. - PubMed
Iaffaioli 1988 {published data only}
-
- Iaffaioli RV, Frasci G, Tortora G, Ciardiello F, Nuzzo F, Scala S, et al. Effect of thymic extract "thymostimulin" on the incidence of infections and myelotoxicity during adjuvant chemotherapy for breast cancer. Thymus 1988;12:69‐75. - PubMed
Kreuser 1998 {published data only}
-
- Kreuser ED. [Randomised, double‐blind trial to compare low‐molecular thymic peptides, 5‐fluorouracil and folic acid with placebo, 5‐fluorouracil and folic acid in patients with metastasised colorectal cancer] [Randomisierter, doppelblinder Therapievergleich von niedermolekularen Thymus‐ Peptiden, 5‐Fluorouracil und Folinsäure versus Placebo, 5‐Fluorouracil und Folinsäure bei Patienten mit metastasiertem kolorektalen Karzinom im Stadium UICC IV (pT1‐4, N0‐2, M1)]. Internet ("This trial was identified via internet databases. In this multicenter study patients were randomised to receive thymuvocal or placebo. The pharmaceutical company (DR Schmidt) supposed to be in charge of did not provide information about this study".) 1998.
Liberati 1998 {published data only}
Mantovani 1995 {published data only}
-
- Mantovani G, Ghiani M, Bianchi A, Curreli L, Contini L, Maccio A, et al. [The immunological assessment of a combined therapeutic approach (chemo‐ + radiotherapy +/‐ surgery) including immunotherapy in neoplasms of the head and neck area at an advanced stage] [Valutazione immunologica di un approccio terapeutico combinato (chemio + radioterapia +/‐ chirurgia) includente l'immunoterapia nelle neoplasie del distretto cervico‐facciale in stadio avanzato.]. Recenti Progressi in Medicina 1995;86:8‐16. - PubMed
Migeod 1985 {published data only}
-
- Migeod F. [Results of a prospective randomised study on the immunologic effects of the xenogenic organ lysates Ney Tumorin‐Sol and Neythymun compared to placebo] [Ergebnisse einer prospektiv randomisierten Studie über immunologische Wirkungen der xenogenen Organlysate Ney Tumorin‐Sol und Neythymun in Vergleich zu einem Placebo]. Therapiewoche 1985;35, 26A:115‐20.
Munno 1995 {published data only}
Quang‐Xing 2001 {published data only}
-
- Quang‐Xing Ni, Fu DI, Yu XJ, Xu J, Hua JM, Chen W, et al. Efficacy of thymalfasin on cellular immune function and chemotherapy induced toxicity in pancreatic cancer. Proceedings American Society of Clinical Oncology 2001.
Shoham 1988 {published data only}
-
- Shoham J. The effect of the thymic factor thymostimulin on survival rate, immune function and resistance to infection in experimental and clinical cancer. Thymic Hormones in Oncology. Ares Serono Symposia, 1988:45‐57.
Surico 1992 {published data only}
-
- Surico N, Tavassoli K. Effect of immunostimulating therapy on the immunocompetent system in breast carcinoma. Panminerva Medica 1992;34:172‐80. - PubMed
Tetti 1987 {published data only}
-
- Tetti M, Tomassone W, Milani R, Paste M, Vaudano G, Rolfo A, et al. Uso di un farmaco immunomodulatore nella prevenzione della comparsadi episodi infettivi a carico delle vie urinarie in pazienti affette da neoplasie ginecologiche e sottoposte a ciclo TCT. Recenti Progressi in Medicina 1987;78 Suppl 6:51‐3.
References to studies awaiting assessment
Dollinger 2010 {published data only}
Additional references
Billich 2002
-
- Billich A. Thymosin alpha1. SciClone Pharmaceuticals. Current Opinion in Investigational Drugs 2002;3(5):698‐707. - PubMed
Bodey 2000
-
- Bodey B, Bodey BJr, Siegel SE, Kaiser HE. Review of thymic hormones in cancer diagnosis and treatment. International Journal of Immunopharmacology 2000;22(4):261‐73. - PubMed
Cha 2003
-
- Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin beta4 in tumor metastasis and angiogenesis. Journal of the National Cancer Institute 2003;95(22):1674‐80. - PubMed
Chretien 1978
-
- Chretien PB, Lipson SD, Makuch R, Kenady DE, Cohen MH, Minna JD. Thymosin in cancer patients: in vitro effects and correlations with clinical response to thymosin immunotherapy. Cancer Treatment Reports 1978;62(11):1787‐90. - PubMed
Costanzi 1977
-
- Costanzi JJ, Gagliano RG, Delaney F, Harris N, Thurman GB, Sakai H, et al. The effect of thymosin on patients with disseminated malignancies. A phase I study. Cancer 1977;40(1):14‐9. - PubMed
CTC 2009
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) ‐ Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/About.html May 28, 2009.
Dunn 2002
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology 2002;3(11):991‐8. - PubMed
Ernst 1997
-
- Ernst E. Thymus therapy for cancer? A criteria‐based, systematic review [see comments]. European Journal of Cancer 1997;33(4):531‐5. - PubMed
Falchetti 1977
-
- Falchetti R, Bergesi G, Eshkol A, Cafiero G, Adorini L, Caprino L. Pharmacological and biological properties of a calf thymus extract (TP‐1). Drugs Under Experimental and Clinical Research 1977;3:39‐46.
Goldstein 1966
Goldstein 1977
Goldstein 1979
-
- Goldstein G, Scheid MP, Boyse EA, Schlesinger DH, Wauwe J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science 1979;204(4399):1309‐10. - PubMed
Goldstein 2009
-
- Goldstein AL, Goldstein AL. From lab to bedside: emerging clinical applications of thymosin à1. Expert Opinion on Biological Therapy 2009;9(5):593‐608. - PubMed
Grothey 1998
-
- Grothey A, Düppe J, Hasenburg A, Voigtmann R. Anwendung alternativmedizinischer methoden durch onkologische patienten. Deutsche Medizinische Wochenschrift 1998;123:923‐9. - PubMed
Hannappel 2003
-
- Hannappel E, Huff T. The thymosins prothymosin à, parathymosin, and á‐thymosins: structure and function. Vitamins and Hormones. Academic press, 2003:2‐296. - PubMed
Hardell 1998
-
- Hardell L, Nordenstam M, Moqvist I, Lindberg L. A new study of patients with cancer in Umea alternative medicine is no alternative [Ny studie av cancerpatienter i Umea: alternativmedicin inget alternativ.]. Lakartidningen 1998;95(18):2092‐5. - PubMed
Higgins 2009
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Vol. available from www.cochrane‐handbook.org, The Cochrane Collaboration, 2009.
Kirkwood 2003
-
- Kirkwood BR, Sterne JAC. Essential medical statistics. Essential medical statistics. Oxford: Blackwell Science Ltd, 2003:Chapter 22.
Kullmer 1999
-
- Kullmer U, Stenger K, Milch W, Zygmunt M, Sachsse S, Münstedt K. Self‐concept, body image, and use of unconventional therapies in patients with gynaecological malignancies in the state of complete remission and recurrence. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1999;82(1):101‐6. - PubMed
Miller 1981
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting Results of Cancer Treatment. Cancer 1981;47(1):207‐14. - PubMed
Moschen 2001
-
- Moschen R, Kemmler G, Schweigkofler H, Holzner B, Dnser M, Richter R, et al. Use of alternative / complementary therapy in breast cancer patients ‐ a psychological perspective. Supportive Care in Cancer 2001;9:267‐74. - PubMed
Rost 1999
-
- Rost KL, Wierich W, Masayuki F, Tuthill CW, Horwitz DL, Herrmann WM. Pharmacokinetics of thymosin alpha1 after subcutaneous injection of three different formulations in healthy volunteers. International Journal of Clinical Pharmacology and Therapeutics 1999;37(1):51‐7. - PubMed
Schlesinger 1975
-
- Schlesinger DH, Goldstein G, Scheid MP, Boyse EA. Chemical synthesis of a peptide fragment of thymopoietin II that induces selective T cell differentiation. Cell 1975;5(4):367‐70. - PubMed
Schulof 1985a
-
- Schulof RS. Thymic peptide hormones: basic properties and clinical applications in cancer. Critical Reviews in Oncology/Hematology 1985;3(4):309‐76. - PubMed
Sehouli 2000
-
- Sehouli J. How frequently do female patients with gynecological cancers use unconventional cancer therapies?. Gynecologic Oncology 2000;79(2):336‐7. - PubMed
Seybold 1950
-
- Seybold WD, McDonald JR, Clagett OR, Good CA. Tumors of the thymus. Journal of Thoracic Surgery 1950;20(2):195‐215. - PubMed
Singh 1998
-
- Singh VK, Biswas S, Mathur KB, Haq W, Garg SK, Agarwal SS. Thymopentin and splenopentin as immunomodulators. Current status. Immunologic Research 1998;17(3):345‐68. - PubMed
Soellner 1997
-
- Soellner W, Zingg‐Schir M, Rumpold G, Fritsch P. Attitude toward alternative therapy, compliance with standard treatment, and need for emotional support in patients with melanoma. Archives of Dermatology 1997;133(3):316‐21. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

