Lamotrigine for acute and chronic pain
- PMID: 21328280
- PMCID: PMC4161113
- DOI: 10.1002/14651858.CD006044.pub3
Lamotrigine for acute and chronic pain
Update in
-
Lamotrigine for chronic neuropathic pain and fibromyalgia in adults.Cochrane Database Syst Rev. 2013 Dec 3;2013(12):CD006044. doi: 10.1002/14651858.CD006044.pub4. Cochrane Database Syst Rev. 2013. PMID: 24297457 Free PMC article.
Abstract
Background: This is an update of the original Cochrane review published in Issue 2, 2007. Some antiepileptic medicines have a place in the treatment of neuropathic pain (pain due to nerve damage). This updated review adds five new additional studies looking at evidence for Lamotrigine as an effective treatment for acute and chronic pain.
Objectives: To assess analgesic efficacy and adverse effects of the antiepileptic drug lamotrigine in acute and chronic pain.
Search strategy: Randomised controlled trials (RCTs) of lamotrigine in acute, and chronic pain (including cancer pain) were identified from MEDLINE, EMBASE and CENTRAL up to January 2011. Additional studies were sought from the reference list of the retrieved papers.
Selection criteria: RCTs investigating the use of lamotrigine (any dose, by any route, and for any study duration) for the treatment of acute or chronic pain. Assessment of pain intensity or pain relief, or both, using validated scales. Participants were adults aged 18 and over. Only full journal publication articles were included.
Data collection and analysis: Dichotomous data (ideally for the outcome of at least 50% pain relief) were used to calculate relative risk with 95% confidence intervals. Meta-analysis was undertaken using a fixed-effect model. Numbers needed to treat to benefit (NNTs) were calculated as the reciprocal of the absolute risk reduction. For unwanted effects, the NNT becomes the number needed to harm (NNH) and was calculated.
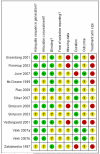
Main results: Twelve included studies in 11 publications (1511 participants), all with chronic neuropathic pain: central post stroke pain (1), chemotherapy induced neuropathic pain (1), diabetic neuropathy (4), HIV related neuropathy (2), mixed neuropathic pain (2), spinal cord injury related pain (1), and trigeminal neuralgia (1); none investigated lamotrigine in acute pain. The update had five additional studies (1111 additional participants). Participants were aged between 26 and 77 years. Study duration was 2 weeks in one study and at least 6 weeks in the remainder; eight were of eight week duration or longer. There is no convincing evidence that lamotrigine is effective in treating acute or chronic pain at doses of about 200-400 mg daily. Almost 10% of participants taking lamotrigine reported a skin rash.
Authors' conclusions: The additional studies tripled participant numbers providing data for analysis, and new, more stringent criteria for outcomes and analysis were used; conclusions about lamotrigine's lack of efficacy in chronic pain did not change. Given availability of more effective treatments including antiepileptics and antidepressant medicines, lamotrigine does not have a significant place in therapy based on available evidence.
Figures
Update of
-
Lamotrigine for acute and chronic pain.Cochrane Database Syst Rev. 2007 Apr 18;(2):CD006044. doi: 10.1002/14651858.CD006044.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Feb 16;(2):CD006044. doi: 10.1002/14651858.CD006044.pub3. PMID: 17443611 Updated.
References
References to studies included in this review
-
- Eisenberg E, Lurie Y, Breker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. Neurology. 2001;57(3):505–9. - PubMed
-
- Lurie Y, Brecker C, Daoud D, Ishay A, Eisenberg E. Lamotrigine in the treatment of painful diabetic neuropathy: a randomized placebo controlled study. Progress in Pain Research and Management. 2000;16:857–62.
-
- Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. 2002;96(3):375–83. DOI: 10.1016/S0304-3959(01)00484-5. - PubMed
-
- Jose VM, Bhansali A, Hota D, Pandhi P. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabetic Medicine. 2007;24:377–83. DOI: 10.1111/j.1464-5491.2007.02093.x. - PubMed
-
- McCleane G. 200 mg daily of lamotrigine has no analgesic effect in neuropathic pain: a randomised, double-blind, placebo controlled trial. Pain. 1999;83(1):105–7. DOI: 10.1016/S0304-3959(99)00095-0. - PubMed
References to studies excluded from this review
-
- Bonicalzi V, Canavero S, Cerutti F, Piazza M, Clemente M, Chio A. Lamotrigine reduces total postoperative analgesic requirement: a randomized double-blind, placebo-controlled pilot study. Surgery. 1997;122(3):567–70. DOI: 10.1016/S0039-6060(97)90129-X. - PubMed
-
- Breuer B, Pappagallo M, Knotkova H, Guleyupoglu N, Wallenstein S, Portenoy RK. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clinical Therapeutics. 2007;29:2022–30. DOI: 10.1016/j.clinthera.2007.09.023. - PubMed
-
- Carrieri PB, Provitera VV, Lavorgna L, Bruno R. Response of thalamic pain syndrome to lamotrigine. European Journal of Neurology. 1998;5(6):625–6. - PubMed
-
- Devulder J, De Laat M. Lamotrigine in the treatment of chronic refractory neuropathic pain. Journal of Pain Symptom Management. 2000;19(5):398–403. DOI: 10.1016/S0885-3924(00)00131-7. - PubMed
-
- di Vadi PP, Hamann W. The use of lamotrigine in neuropathic pain. Anaesthesia. 1998;53(8):808–9. DOI: 10.1046/j.1365-2044.1998.00564.x. - PubMed
Additional references
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, characteristics and mechanisms. European Journal of Pain. 2006;10(4):287–333. DOI: 10.1016/j.ejpain.2005.06.009. - PubMed
-
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain. 2008;9(2):105–21. DOI: 10.1016/j.jpain.2007.09.005. - PubMed
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology. 2002;31:140–149. DOI: 10.1093/ije/31.1.140. - PubMed
References to other published versions of this review
-
- Wiffen PJ, Rees J. Lamotrigine for acute and chronic pain. Cochrane Database of Systematic Reviews. 2007;(2) DOI: 10.1002/14651858.CD006044.pub2. - PubMed
-
-
* Indicates the major publication for the study
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical